Exploring how treatments targeting LDL-C are redefining the fight against Atherosclerotic Cardiovascular Disease (ASCVD), the leading cause of death worldwide.
Atherosclerotic cardiovascular disease (ASCVD) remains the major cause of death worldwide, responsible for over 18 million deaths annually.1
Although risk factors for ASCVD disease are multifactorial there is overwhelming evidence that hypercholesterolaemia, particularly elevated levels of LDL-cholesterol (LDL-C), is the pivotal causal risk factor.2 Data that has accrued from genetic studies, observational data and large, randomised clinical trials all support a causal contribution of LDL-C to atherosclerosis and ASCVD.3 Although other lipoproteins such as remnants and lipoprotein(a) are also pro-atherogenic, LDL particles constitute over 90% of circulating apolipoprotein-B lipoproteins in the fasting state, so are by far the most important contributor to ASCVD.2 For these reasons, current guidelines all emphasise lipid modification, particularly aimed at reducing LDL-C to reduce ASCVD risk, and LDL-C lowering has become the cornerstone of ASCVD prevention.4
Statins remain the first choice of lipid lowering treatment. Findings from large, randomised statin trials involving over 170 000 participants have conclusively demonstrated that for every 1mmol/L reduction in LDL-C there is a 20%-25% reduction in the incidence of myocardial infarction and of ischaemic stroke and a 12% reduction in total mortality.5 Consequently, statins are now being used by millions of people worldwide. However, statins alone do not eliminate ASCVD, and ‘residual risk’ remains. In a recent meta-analysis the reduction in cardiovascular risk for every 1mmol/L reduction in LDL-C was independent of drug class, implying that the benefit from statins and other non-statin drugs such as ezetimibe and PCSK9 inhibitors are probably derived from the LDL-C lowering effect of these drugs rather than from any pleotropic effects.6 It is also important to emphasise that the absolute benefit of lowering LDL-C also depends both on the absolute risk for ASCVD and on the reduction in LDL-C achieved. Even a small absolute reduction in LDL-C can translate into a significant absolute reduction in patients at very high cardiovascular risk, such as patients who have suffered a recent acute coronary syndrome who are at extremely high risk of recurrent events.7 In addition, since patients with more risk factors for ASCVD such as diabetes mellitus, hypertension, central obesity and tobacco smoking, have a higher absolute risk of ASCVD, reduction in LDL-C will translate into a greater absolute risk reduction in these patients.
Lower is better but LDL-C goal achievement is getting increasingly more difficult particularly in high-risk patients in whom the recommended LDL-C treatment target is now <1.4mmol/L or even 1mmol/L in patients with established ASCVD with recurrent events. The treatment target of <1.4mmol/L is based on data from genetic Mendelian randomisation studies and from meta-analysis of large lipid lowering randomised controlled trials.4 Guidelines also recommend a LDL-C reduction of at least 50% from the pre-treatment baseline value.4 (Figure 1).
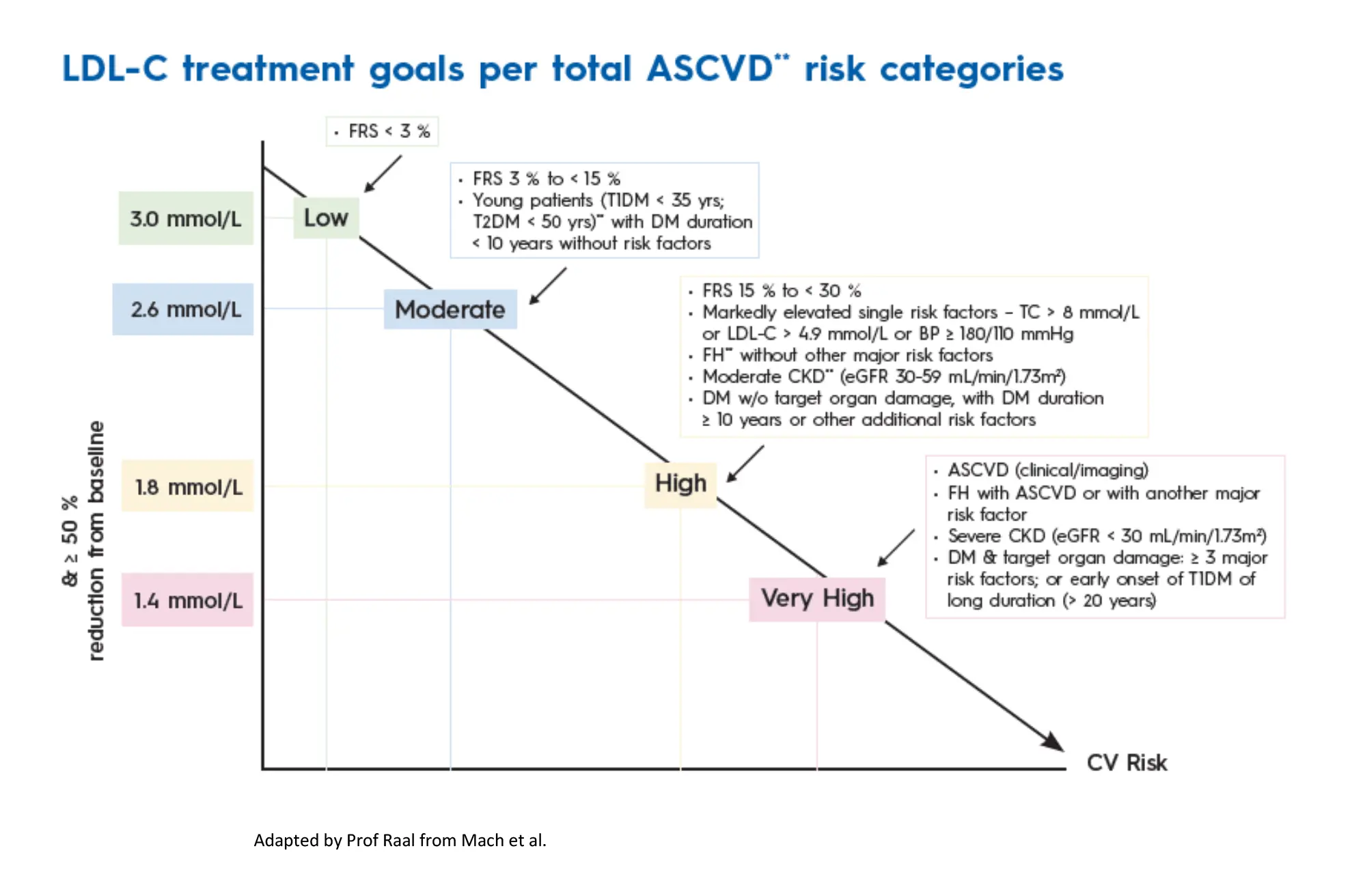
This is based on the observation that percent LDL-C reduction provided incremental benefit over attained LDL-C, particularly in patients with established ASCVD.7 In a recent study of over 40 000 patients followed for a median of 3.8 years, greater LDL-C reduction with more intensive lipid-lowering therapy after an acute myocardial infarction was associated with a reduced hazard for all CV outcomes as well as a reduction in all-cause mortality.8 More recent studies have also shown that the benefit of LDL-C lowering is the highest when very low levels of LDL-C are achieved. In a recent meta-analysis of over 100 000 patients, very low LDL-C levels obtained with intensive lipid-lowering treatments were not associated with any major adverse events but resulted in a persistent reduction of cardiovascular events with lower LDL-C levels.9 There is thus currently no level of LDL-C below which further benefit is not accrued and no signal of harm from low LDL-C levels so, ‘the lower, the better’.
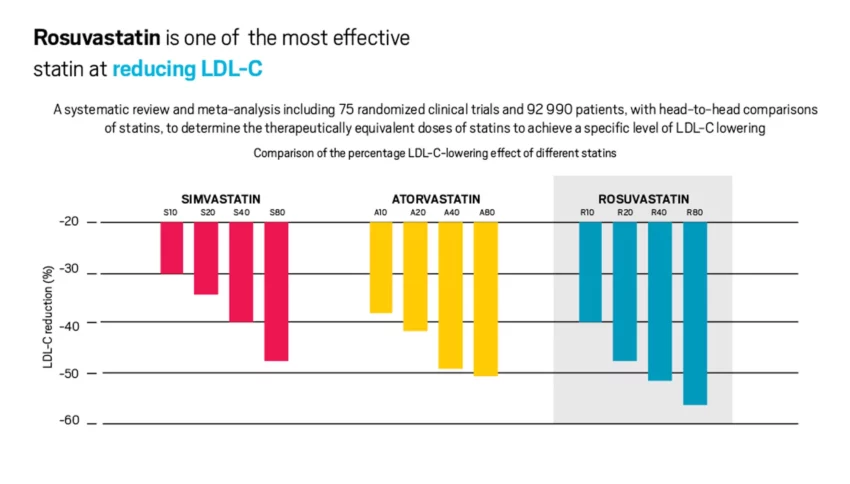
However, despite the guidelines, most patients remain untreated or undertreated and guideline recommended LDL-C targets are rarely achieved as shown in the latest EUROSPIRE survey conducted in 27 countries in Europe.10 The reasons behind this failure to achieve guideline recommended LDL-C targets are multiple and include perceived side effects, particularly from high-intensity statins, lack of patient and physician education as well as patient adherence. Non-adherence to statin therapy is as high as 60% and is associated with an increased risk of CV events.11 A recent meta-analysis of the large-scale, randomised double-blind statin studies of over 120 0000 participants showed that only one in 15 cases of statin-associated muscle symptoms were caused by the statin.12 The small risk of muscle symptoms is insignificant compared to the highly proven cardiovascular benefit of statin therapy. Nevertheless, patients (and physicians) are still concerned about the side-effects of high-intensity statin therapy and one solution is to consider lower-intensity statin together with ezetimibe. The maximum dose of a high intensity statin such as atorvastatin or rosuvastatin should be used (Figure 2), but if this is not tolerated greater reduction in LDL-C can be attained with the combination of a lower dose of statin together with ezetimibe.
Real-world data has shown poor achievement of guideline recommended LDL-C targets and very limited use of combination lipid-lowering therapies. For example, in the DA VINCI registry conducted in Europe, most patients with established ASCVD only received moderate-intensity statin monotherapy (44%) and fewer high-intensity statin (38%). Only 22% of high-risk secondary prevention patients were at LDL-C target and only 9% of patients were on a combination of a statin with ezetimibe and less than 1% on a combination including a PCSK9 monoclonal antibody.13 Similarly, in the GOULD registry conducted in the US, only 17% of patients with established ASCVD and LDL-C levels above target had their lipid lowering medications intensified, and the addition of ezetimibe was only considered in 5% of patients.14 This inertia could be overcome, or at least improved through early initiation of combination lipid lowering treatment, which will result in a greater proportion of patients meeting LDL-C targets.
Current guidelines for the management of hypercholesterolaemia in patients at high risk for ASCVD or with established ASCVD recommend monotherapy with the highest tolerated dose of statin before consideration of additional non-statin therapy to reach LDL-C goal.4 However, combination of lipid-lowering therapy reduces LDL-C concentrations to a greater extent than doubling or even trebling the statin dose. Every doubling of a statin dose will lower LDL-C by only a further 6% on average, whereas the addition of ezetimibe with lower LDL-C level by a further 15%-20%.15
Two large, randomised studies, the IMPROVE-IT and the HIJ-PROPER study, have reported on the additive effect of ezetimibe with a same-dose statin regimen but did not include statin dose reductions.16,17 Other studies have compared the combination of low-intensity statin plus ezetimibe to high-intensity statin alone but were of short duration.18 The most potent combination to lower LDL-C is a combination of rosuvastatin plus ezetimibe.19,20 The recently reported RACING TRIAL (randomised comparison of efficacy and safety of lipid lowering with statin monotherapy vs statin-ezetimibe combination for high-risk cardiovascular disease) is the first study to investigate the clinical efficacy and safety of a combination strategy with moderate intensity statin plus ezetimibe compared to high-intensity statin alone.21 The combination therapy resulted in a greater reduction in LDL-C while reduction in cardiovascular events was similar with both treatment arms. A higher proportion of subjects achieved an LDL-C concentration of less than 1.8mmol/L and there was a lower prevalence of drug discontinuation or dose reduction caused by intolerance to study drug in the combination treatment group. This important study paves the way to consider the early initiation of combination therapy particularly in patients at high ASCVD risk and in those not tolerating high-intensity statins. Aggressive lipid-lowering therapy is also especially important for patients with familial hypercholesterolaemia (FH), the most common dominantly inherited condition affecting 1 in approximately 300 persons worldwide.22 As a result of a founder effect, the prevalence of FH is even higher, about 1 in 70, in certain population groups in SA, such as our Afrikaans, Jewish and Asian populations.23,24 If untreated, the arteries of patients with FH are exposed to double the amount of circulating LDL-C from birth. By the age of 40 years, their arteries would have been exposed to the same amount of LDL-C as a healthy 80-year-old.
As ASCVD are the result of cumulative exposure to LDL-C it is also important to initiate treatment as early as possible. So not only ‘the lower the better’, but ‘the earlier the better’, particularly in patients with FH.25 It is therefore important to test family members, particularly the children, when a diagnosis of FH is suspected. Current recommendations are to start lipid-lowering therapy in both boys and girls diagnosed with FH at the age of eight years, in order to reduce the cumulative burden of LDL-C over time.26 A recently published study from the Netherlands has shown that the risk of ASCVD can be reduced to that of the background health population if lipid-lowering therapy is initiated in childhood in subjects with FH.27 In addition, as a result of high untreated LDL-C levels, guideline recommended LDL-C targets are infrequently achieved with single-drug therapy in patients with FH, and combination therapy and earlier introduction of these therapies is essential in order to reduce the global cardiovascular burden of FH.22 To achieve the lower LDL-C targets now recommended, we need to revise our treatment strategy for the management of hypercholesterolaemia, as has occurred for the treatment of hypertension over the past two decades. Single agent therapy is no longer an option for the treatment of essential hypertension. The hypertension guidelines changed way back in in 2003, with initial consideration of combination therapy, particularly for more severe elevations of blood pressure.28
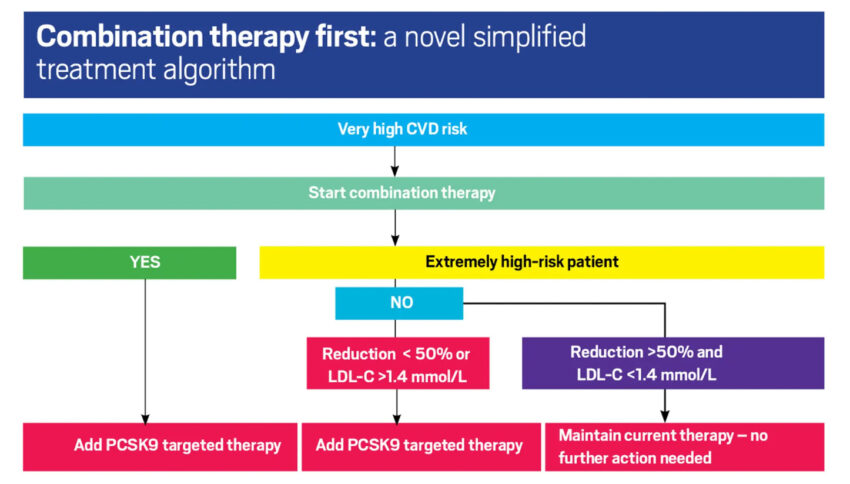
Combination therapy, for example an ACE inhibitor plus diuretic or an ACE inhibitor plus a calcium-channel blocker has become the norm. The same rationale should be considered when lipid lowering therapy is initiated. In patients with marked hypercholesterolaemia, such as patients with FH, and particularly in those with established ASCVD, first-line therapy should be a statin in combination with ezetimibe.29 We need to move away from a ‘high-intensity statin’ concept to a tailored ‘lipid-lowering strategy’ using combinations of lipid-lowering drug therapies as first-line therapy particularly for our high-risk patients.30 (Figure 3).
References available on request.








