SA Ophthalmology Journal
Earn 3 CPD points

The official journal of the Ophthalmological Society of South Africa
MICROBIAL PROFILE OF VITREOUS AND AQUEOUS ASPIRATES IN PATIENTS WITH ENDOPHTHALMITIS AT INKOSI ALBERT LUTHULI CENTRAL HOSPITAL: A RETROSPECTIVE STUDY
L Shelembe, C Kruse
THE CLINICAL PROFILE OF CHILDHOOD BLINDNESS IN A TERTIARY SOUTH AFRICAN HOSPITAL - A 12-YEAR REVIEW
Z Alashhab, D Minnies, C Tinley
THE UTILITY OF A NON-MYDRIATIC FUNDUS CAMERA IN A TERTIARY DIABETES CLINIC
S Ben Barka, C Laurence, L Du Toit-De Wet, M Conradie-Smit
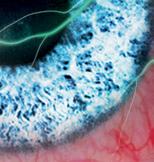
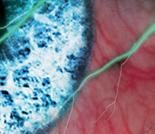
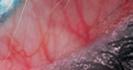
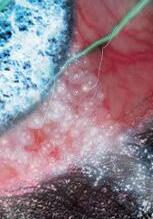
FROSTED BRANCH ANGIITIS: A RARE BLINDING VASCULITIS IN THREE CHILDREN PRESENTING ACUTELY TO RED CROSS CHILDREN’S WAR MEMORIAL HOSPITAL… UNMASKING POSSIBLE MUMPS-ASSOCIATED SEQUELAE IN THE UNVACCINATED? N Narainswami, N Freeman, T Seobi
PENETRATING ORBITAL INJURY CAUSING A PRE-PONTINE HAEMORRHAGE AND ABDUCENS PALSY
S Rashid, B Kgaodi, C Tinley, N Enslin, A Figaji
| Vol
• No
AUTUMN 2024
19
2
Editor-in-Chief Prof Nagib du Toit journaleditor@ossa.co.za
Assistant editors
Prof Christopher Tinley christopher.tinley@uct.ac.za
Dr Naseer Ally naseerally@gmail.com Managing Editor Gill Abrahams | 082 330 9540 Gill.Abrahams@newmedia.co.za
Expert Board
Professors: Colin Cook, Nagib du Toit, Priscilla Makunyane, Aubrey Makgotloe, Hamzah Mustak, Christopher Tinley, Linda Visser, Susan Williams
Doctors: Eric Albrecht, Hassan Alli, Naseer Ally, Stephen Cook, Leonard Heydenrych, Roland Hollhumer, Mpopi Lenake, Stephen Manyeruke, James Rice, Tshilidzi van der Lecq Subscriptions Felicity Garbers

3 https://www.medicalacademic.co.za/brand/ophthalmology-journal-of-south-africa/ www.medicalacademic.co.za
Ophthalmology Journal
official
of the Ophthalmological
of South
ISSN:
SA
The
journal
Society
Africa
2218-8304
Layout & Design: Allison McCallum Advertising Sales Charissa Piek | 063 281 1205 charissa.piek@newmedia.co.za
incl. VAT R80,00 per annum Publishing Team General Manager: Dev Naidoo Head of Commercial: B2B & Owned Brands: Johann Gerber Johann.Gerber@newmedia.co.za Production Manager: Angela Silver Art Director: David Kyslinger Digital Manager: Varushka Padayachi Contact New Media Johannesburg Ground Floor, 272 Pretoria Avenue, Randburg 2194 Tel: 011 877 6111 Fax: 011 713 9024 Postal Address: PO Box 784698, Sandton 2146 www.medicalacademic.co.za Printing: Printed and bound by CTP Printers Published by New Media, a division of Media24 (Pty) Ltd Management team CEO: NewMedia: Aileen Lamb Commercial Director: Maria Tiganis Strategy Director: Andrew Nunneley Chief Financial Officer: Venette Malone CEO: Media24: Ishmet Davidson Head office 8th floor, Media24 Centre, 40 Heerengracht, Cape Town 8001 PO Box 440, Green Point, Cape Town 8051 Tel: +27 (0)21 406 2002 www.newmedia.co.za The reproduction, without permission of any articles or photographs in this publication is forbidden and copyright is expressly reserved to NewMedia under the Copyright Act of 1978 as amended. The views expressed by contributors to and advertisers in the journal and the inclusion or exclusion of any medicine or procedure, do not necessarily reflect the views of the publisher or editorial board. While every effort is made to ensure accurate reproduction, the authors, advisers, publishers and their employees or agents shall not be responsible or in any way liable for errors, omissions or inaccuracies in the publication, whether arising from negligence or otherwise or for any consequences arising therefrom. Contents 4 FROM THE EDITOR MMed mission impossible C Tinley 6 GUIDELINES FOR AUTHORS 8 ORIGINAL STUDY Microbial profile of vitreous and aqueous aspirates in patients with endophthalmitis at Inkosi Albert Luthuli Central Hospital: a retrospective study L Shelembe, C Kruse 14 ORIGINAL STUDY The clinical profile of childhood blindness in a tertiary South African hospital - a 12-year review Z Alashhab. D Minnies, C Tinley 18 ORIGINAL STUDY The utility of a non-mydriatic fundus camera in a tertiary diabetes clinic S Ben Barka, C Laurence, L Du Toit-De Wet, M Conradie-Smit
CASE SERIES
branch angiitis: a rare blinding vasculitis in three children presenting acutely to Red Cross Children’s War memorial hospital… unmasking possible mumps-associated sequelae in the unvaccinated?
Narainswami, N Freeman, T Seobi
CASE REPORT
orbital injury causing a pre-pontine haemorrhage and abducens palsy
Rashid, B Kgaodi, C Tinley, N Enslin, A Figaji
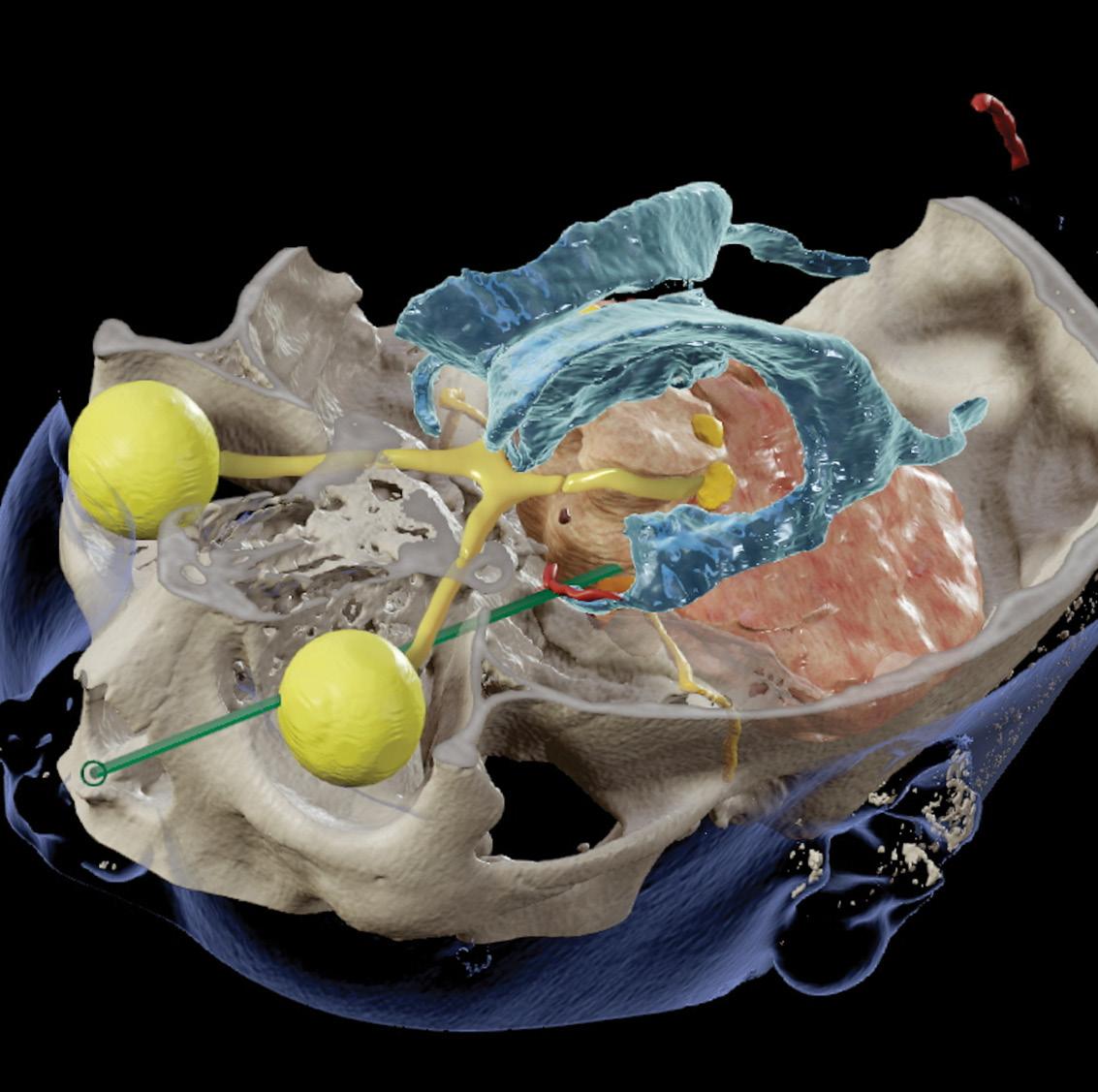
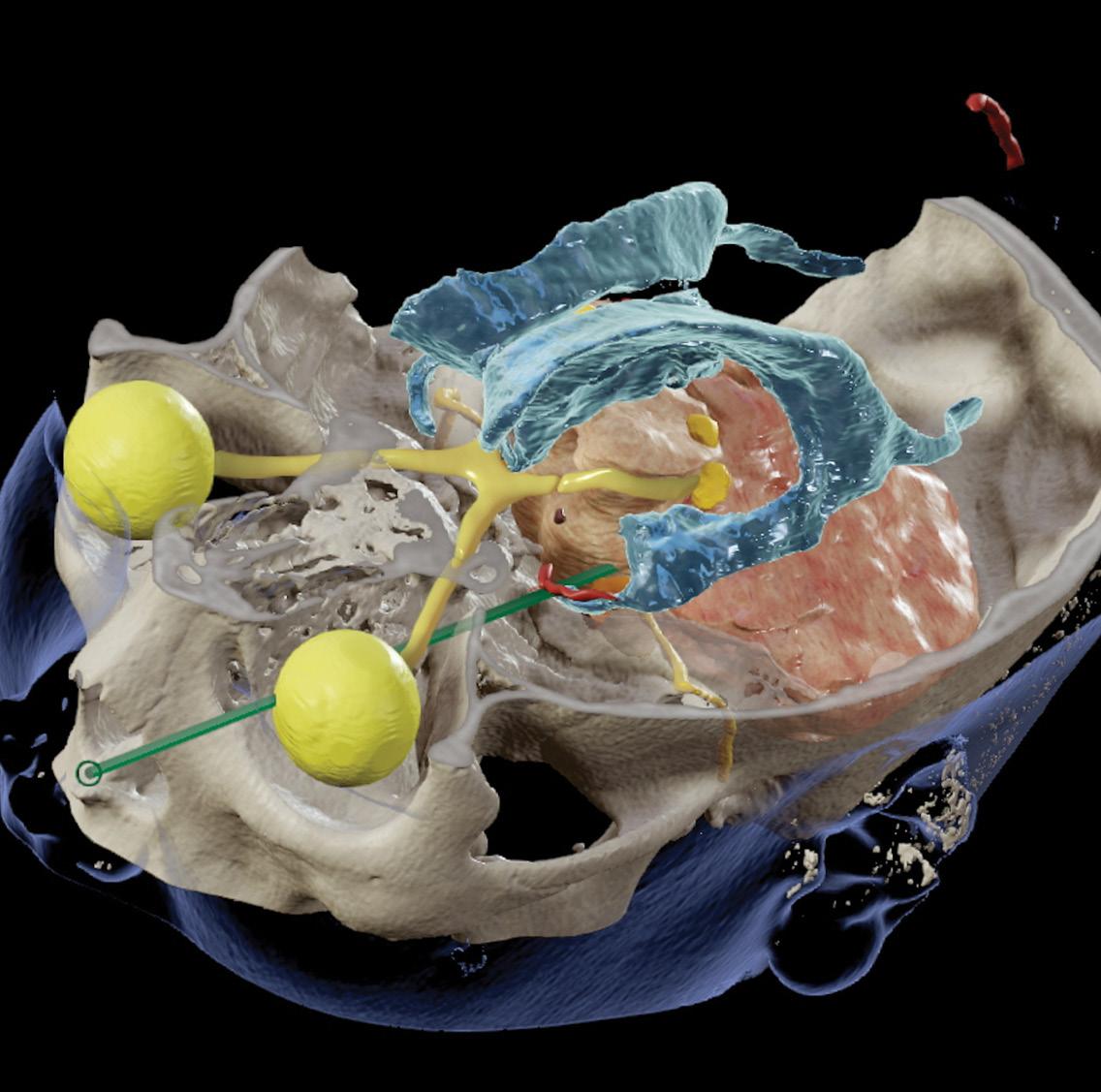
2024
19 • No 2 COVER PIC:
reconstruction demonstrating critical neural and vascular structures in close proximity to the tract.
felicity.garbers@newmedia.co.za Local
25
Frosted
N
30
Penetrating
S
Autumn
Vol
Image

“HMMed Mission Impossible
ow is your MMed going?” is a question that tweaks the adrenals of most South African registrars. Of course, there are always one or two outliers, who breezily bash out their dissertation in the first year, then go on to amuse themselves with a PhD for the remainder of their training. But for the rest of us mortals, the MMed project is a millstone and a long, hard slog.
In December 2015, the HPCSA issued a ruling that completion of a ‘Research Component’ during specialist training was a prerequisite for registration. This created a tsunami of additional academic administrative processes, which engulfed postgraduate offices, institutional supervisors and unsuspecting trainees. Neatly set out in the South African Committee of Medical Deans (SACOMD) document of 12 September 2017, ‘Standardisation of the Research Component of the Master of Medicine Degree - Final Recommendations’, the purpose of this component is said to be fourfold:
1. To stimulate the interest of the student in research
2. To allow students to become familiar with the knowledge and skills which underpin research
3. To stimulate the critical thinking and high-order reasoning required of a level 9 qualification in terms of the HEQSF
4. To promote scholarship within what is otherwise a technical vocational training programme
While in principle this sounds awesome, was it ever feasible? Parking Everest next to a full-time technical vocational training programme was an audacious move indeed.
SACOMD admits that since the inception of the HPCSA rule, there has been little uniformity across universities in terms of the total credits assigned to the MMed degree, the required scope, standard and credit weighting of the research component, and the provision of protected time for performance of research. ‘Supervisors and programme coordinators need to understand their role in ensuring that both informative and transformative learning occur. It is insufficient to restrict supervision to the direction of a series of sequential mechanical activities in the absence of transformative learning. However, preparation of the dissertation itself should not be unnecessarily onerous or time-consuming.
Notional hours
Up to 900 hours of activity are necessary for these learning outcomes to be met. This represents approximately 23 working week equivalents. From a practical standpoint, it would seem reasonable for this time to be made available to the student as 8-12 weeks of dedicated time (whether taken en bloc or distributed over the four years), with the remaining 50% to be undertaken alongside other activities or during own time, i.e. self-directed learning. Time devoted to the research component is effectively time removed from the clinical training component. It is essential that a balance is found which enables the purposes of the research component to be met, while not jeopardising the clinical competence of the graduating specialist.’
Let’s be frank, this balance is rarely found. In our current climate of financial austerity, with reduced healthcare workforces and increased clinical service demands, are we surprised to find our registrars at the end of their training time dangling from a wire, six inches above a floor of laser beams, with beads of sweat dripping from their foreheads?
SACOMD go on to recommend that support should be provided for the research component:
1. ‘It is critical that support structures are put in place to assist the large numbers of MMed students who are now subject to the requirement to perform the research component.
2. The major steps to be undertaken are identified as follows:
Build an environment with a strong research ethos
Nurture and support the research activities of the MMed students
Enhance supervisor capacity
Provide protected research time’ These steps are major indeed and I suspect we are all lagging behind. UCT only introduced formal registrar teaching on the principles and practice of research earlier this year. Many supervisors who have been roped in to help out, have very little research experience themselves.
And as for protected research time, what is that?
Last, SACOMD encourages us to foster enthusiasm for research. ‘It is highly desirable that the graduate emerges from the research component with a respect and
enthusiasm for research, such that he or she is likely to promote the research agenda in the future, whether by personal involvement, or by support and understanding for the work of others.’
In reality, registrars who haven’t entirely submerged, are more likely to emerge from the research component with flashbacks and post-traumatic stress disorders. As supervisors, we can’t always be their Ethan Hunt, a highly skilled field agent for the Impossible Mission Force, who handles dangerous and high-stakes missions. But we can be kind, patient and supportive to all our trainees trying to conquer their MMed Everests.
The SAOJ serves as an invaluable platform to showcase the hard work of registrars, which may not otherwise enjoy visibility in higher-impact international journals. The fact that it is Department of Higher Education and Training (DHET)-accredited, means that some universities like WITS are satisfied to award the MMed degree for a paper published in the journal, without sending it on for further examination.
One of our international registrars was most recent to summit, and his dissertation on The Profile of Childhood Blindness in Cape Town finally rests in this issue. He started in the foothills with a literature review of the topic in May 2021. It may easily have taken 23 working week equivalents, but the majority of this would have been conducted alongside other activities or during his own time. Nevertheless, he is destined for new heights and is busy preparing for a vitreoretinal fellowship expedition in British Columbia in June. I hear the mountains around Whistler are challenging, but spectacular that time of year.

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 4 From the Editor
FRCOphth(London)
Prof Christopher Tinley
Assistant editor: South African Ophthalmology Journal

Vacuklav® 41 B+ Evolution
Fast has never been this fast before in Ophthalmology.
Sterilise more instruments in significantly less time, all while guaranteeing unparalleled reliability. The esteemed Evolution Series by MELAG is unmatched in speed and efficiency.


8 – 21 Min.
Sterilisation times of Vacuklav ® 41 B+ Evolution
20 – 45 Min.

2,5x Faster
Sterilisation times of autoclaves without Double-Jacket Technology
Want to save even more time?

High loading capacity for up to 9 kg ophthalmic instruments on 8 trays or 4 MELAstore Boxes.
Record-breaking sterilisation times thanks to patented Double-Jacket Technology for a quick turnaround time.

Algorithm-controlled DRYtelligence drying for optimal results and 80 % shorter drying times.
XXL colour-touch Display for magical operation and complete documentation as well as approval at the display without an additional computer.
T he MELA store® system consisting of MELA store® Tray and MELA store® Box is the best way to optimise your instrument workflow from treatment to decontamination and storage. T + 27 (0) 10 007 2431 info
@envisionafrica.co.za
South African Ophthalmology Journal guidelines for authors
The SA Ophthalmology Journal is a peerreviewed scientific journal and the official mouthpiece of the Ophthalmological Society of South Africa. It appears on a quarterly basis.
1. The South African Ophthalmology Journal invites review articles, original studies and case reports for submission. Articles should be the original, unpublished work of the stated author. All materials submitted for publication must be submitted exclusively for publication in this journal. Written permission from the author or copyright holder must be submitted with previously published figures, tables or articles. Authors are solely responsible for the factual accuracy of their work.
2. A cover sheet is to be submitted with each manuscript. It should contain the title of the manuscript, the names of all authors in the correct sequence, their academic status and affiliations. The ORCID ID number for each author should be supplied (https:// orcid.org/). The corresponding author should include his/her name, address, phone and email address.
3. Articles should be between 2 000 and 3 000 words in length. A 200-word abstract should state the main conclusions and clinical relevance of the article. Use the headings Background, Methods, Results and Conclusion. Five keywords are to be supplied at the end of the abstract.
4. Authors should declare any interests, financial or otherwise, regarding the publication of their article, under the headings of Funding and Conflict of interest. If none, this should be stated. An ethics statement regarding patient consent and/ or Ethics Board approval should be included. Authors should also indicate whether the submission forms part of an ‘MMed dissertation by publication’ by stating so clearly on the title page.
5. All articles are to be in English and are to
follow the Vancouver style of referencing. References should be numbered consecutively in the order that they are first mentioned in the text and listed at the end in numerical order of appearance. Identify references in the text by Arabic numerals in superscript after punctuation, e.g. … trial.13
6. The following format should be used for references: Articles: Kaplan FS, August CS, Dalinka MK. Bone densitometry observation of osteoporosis in response to bone marrow transplantation. Clin Orthop 1993;294:173-78. Chapter in a book: Young W. Neurophysiology of spinal cord injury. In: Errico TJ, Bauer RD, Waugh T (eds). Spinal Trauma. Philadelphia: JB Lippincott; 1991: 377-94.
7. Tables should carry Roman numerals, I, II etc., and illustrations Arabic numbers 1, 2 etc.
8. Abbreviations and acronyms should be defined on first use and kept to a minimum.
9. All figures, tables and photographs should also be submitted electronically. Each figure must have a separate self-explanatory legend. The illustrations, tables and graphs should not be embedded in the text file, but should be provided as separate individual graphic files, and clearly identified. Photographs should be saved as a 300 dpi JPEG file. Graphs and algorithms, which need to be editable, should be saved as MS Word documents or in PowerPoint. Tables should be saved either in MS Word or in a PowerPoint document. Photographs and X-rays need to be suitably anonymised. Permission should be obtained for the use of patient photographs.
10. Articles are to be submitted by email to the Editor-in-Chief, Prof Nagib du Toit at the following email address: journaleditor@ ossa.co.za The text should be in MS Word. Pages should be numbered consecutively in the following order wherever possible: Title page, abstract, introduction, materials
and methods, results, discussion, acknowledgements, tables and illustrations, references.
11. The Editor reserves the right to shorten and stylise any material accepted for publication.
12. For all accepted articles, authors will be requested to provide five (5) multiple choice CPD questions related to their paper.
13. Authors need to disclose whether they used artificial intelligence (AI)-assisted technologies (such as Large Language Models, chatbots, or image creators) in the production of submitted work. Authors who use such technology should describe, in both the cover letter and the submitted work, how they used it. Authors should not list AI and AI-assisted technologies as an author or co-author, nor cite AI. Chatbots (such as ChatGPT) should not be listed as authors because they cannot be responsible for the accuracy, integrity, and originality of the work. Authors should carefully review and edit the result because AI can generate authoritative-sounding output that can be incorrect, incomplete, or biased and all plagiarism that may have been produced by the AI, should be excluded.
14. Authors are to insert the following copyright notice on their article submissions:
Copyright © 2022 [insert the Author(s) name(s)].
All rights reserved. Copyright subsists in the Author of this work. No part of this article or included photographs may be reproduced, published, performed, broadcast, transmitted or adapted in any form or by any electronic, mechanical or other means without the written permission of the copyright holder. This article is published by New Media, a division of Media24 (Pty) Ltd with consent of the Author. Any unauthorised reproduction, publishing, or adaption of this work will constitute copyright infringement and render the doer liable under both civil and criminal law.
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 6 Guidelines for authors
The CPD questions now have to be completed online. To complete the questionnaire, go to https://www.medicalacademic.co.za/courses/ sa-ophthalmology-journal-cpds-autumn-2024

Microbial profile of vitreous and aqueous aspirates in patients with endophthalmitis at Inkosi Albert Luthuli Central Hospital: a retrospective study
L Shelembe, MBChB (UKZN); Registrar, Department of Ophthalmology, University of KwaZulu-Natal
https://orcid.org/0009-0008-5497-4595
C Kruse, MBChB (UP), MMed (Ophth) UKZN, FCOphth (SA); Academic Head of Department, Department of Ophthalmology, University of KwaZulu-Natal
https://orcid.org/0000-0002-8805-8383
Corresponding author : Dr L Shelembe, e-mail: lindelani.shelembe@gmail.com
Abstract
Background: Endophthalmitis is a rare but challenging condition to manage. It can result in significant and often permanent visual loss. Local microbial and sensitivity analyses are important to direct treatment guidelines.
Methods: A retrospective analysis of patients with clinically diagnosed endophthalmitis who had either a vitreous or aqueous specimen submitted between January 2016 and December 2022. Information on age, mechanism of inoculation, and microbial results were captured from the hospital electronic records.
Results: Most of our cases were from post-surgical endophthalmitis (n = 11; 37.9%) and post-traumatic (n = 9; 31.0%) followed by endogenous (n = 5; 17.2%), and post-intravitreal injection (n = 4; 13.8%).
The most common organism group cultured was Grampositive cocci (n = 15; 83%). Within this Gram-positive group, staphylococci were predominant (n = 12; 80%) followed by streptococci (n = 2; 13.3%) and enterococcus (n = 1; 6.7%). Overall
Introduction
Endophthalmitis is ocular inflammation involving the vitreous cavity along with the retinal and uveal components of the eye, mostly due to an infectious agent(s).1 It is a rare, but often challenging disease to manage and can result in significant and often permanent visual loss.
The incidence and mechanism of infectious endophthalmitis vary in different regions. Cataract surgery is by far the most common cause of exogenous
yield was positive in just over half of the samples (n = 15; 51.7%).
Our results showed no case of fungal endophthalmitis. None of the cases in our study showed any resistance to a combination of Vancomycin and Ceftazidime.
Conclusion: Our study demonstrated microbial profiles that are comparable with those of similar studies. We found no evidence of resistance to any of our first-line agents. Intravitreal vancomycin and ceftazidime, therefore, continue to be an appropriate first-line treatment for bacterial endophthalmitis across different ages, causes, and types of inoculation in our population.
Keywords: endophthalmitis, KwaZulu-Natal, microbiology, culture, exogenous, endogenous.
Funding and conflict of interest: none. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
endophthalmitis, with some estimating it to be responsible for 90% of cases. 2 In their study, Tien Yin Wong et al. reported rates of 0.07%-0.32% of exogenous endophthalmitis following cataract surgery in 44 803 patients in the Singapore National Eye Centre. 3 In their study, Chee et al. reported endogenous endophthalmitis as accounting for 2%-8% of as all cases of endophthalmitis also in Singapore.4 Similarly, the proportional spread between endogenous and exogenous endophthalmitis differs between regions,
however, it is a generally accepted phenomenon that the occurrence of exogenous endophthalmitis tends to supersede endogenous endophthalmitis. One of the highest numbers of endogenous endophthalmitis comes from a report by Krause et al., where endogenous endophthalmitis accounted for 41% of cases in London. 5 Ramakrishnan et al. suggested that as much as 92.6% were exogenous in origin in their study of the Indian population.6
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 8 Original study Endophthalmitis microbial profile
A variation exists in microbial spectra between regions, mechanism of inoculation, and duration of exposure to possible inoculation. In the landmark study of the early to mid-1990s, the Early Vitrectomy Study looked at post-operative endophthalmitis, confirmed microbiologic growth showed a strong Gram-positive, coagulase-negative staphylococci predominance.7 This trend was echoed in the French Institutional Endophthalmitis Study group.8 In their study looking at visual outcomes post-surgery, Gupta et al. found a predominance of fungal isolates in cases of exogenous endophthalmitis in India, particularly post-cataract surgery.9 Connell et al. looked at endogenous endophthalmitis at a tertiary referral centre in Australia and also found higher rates of fungal isolates compared to bacterial cultures.10 These all point to varying microbiological trends between regions.
The increasing use of intravitreal injections is a risk factor for inoculation, albeit rare.11 In one study, Fintak et al. found the incidence of endophthalmitis post intravitreal bevacizumab and ranibizumab injection to be 1 in 4500.12 Interestingly, in this large study of more than 26 000 injections, they had only six cases of infectious endophthalmitis; four of them culturing Streptococci species, while the other two showed no growth.
The current empiric antibiotic regimen we use in our local eye centres is intravitreal vancomycin to cover Gram-positive organisms and intravitreal ceftazidime to target Gram-negative bacilli and Pseudomonas. These guidelines are largely influenced by North American practices. 2
In South Africa, and particularly in KwaZulu-Natal, there is a paucity of comprehensive, and clear epidemiological and microbiological studies looking at the occurrence of endophthalmitis, its microbial profile, and susceptibilities.
From a South African perspective, some studies looked at the prevention of endophthalmitis. In particular, Du Toit et al. looked at the role of prophylactic antibiotics in open-globe injuries to prevent endophthalmitis while Van Der Merwe et al. looked at the role of intracameral cefuroxime particularly during cataract surgery for prevention of postoperative endophthalmitis.13,14 There still exists a need to document microbial patterns for our local population to determine prevailing susceptibility trends. Understanding the microbial profile of our region will guide us in determining whether we need to adopt different
empiric antimicrobial protocols. This study aimed to identify the microbial spectrum of endophthalmitis and to determine the antibacterial susceptibilities of microbial isolates of patients treated at Inkosi Albert Luthuli Central Hospital (IALCH), in the subtropical coastal city of Durban, South Africa. This would assist in identifying the most appropriate empirical therapy for infectious endophthalmitis within the province.
Methods
A retrospective analysis of patients with clinically diagnosed endophthalmitis who had either a vitreous or aqueous specimen submitted between January 2016 and December 2022. Information on age, mechanism of inoculation, and microbial results was captured from the hospital’s electronic records.
Samples not taken from clinically confirmed cases of endophthalmitis and samples not marked specifically as vitreous or aqueous specimens, as well as specimens submitted before or after the study period (January 2016 to December 2022), were excluded.
Information relating to patients’ age, sex, mechanism of inoculation, and microbial culture outcome of the samples were received from the IALCH MEDITECH® health record system. This information was
captured on an Excel® data capture tool using a de-identified ‘flat’ database design to facilitate statistical analysis: Each row contained one complete case (anonymized) and each column a specified data field. Missing data was ‘entered’ as empty fields.
Data was analysed was done in SPSS® version 27. Frequencies and percentages were calculated to summarise categorical variables. Central tendency and dispersion of numerical data were measured using means and standard deviations, if these variables are normally distributed, and medians and interquartile ranges if the variables are skewed.
Ethical approval was obtained from the University of KwaZulu-Natal Biomedical Research Committee (BREC/00005284/2023).
Results
Our overall number of cases with endophthalmitis was 29. These cases were seen either by a registrar/resident or medical officer and discussed with a consultant ophthalmologist at the referring base hospital before referral to IALCH.
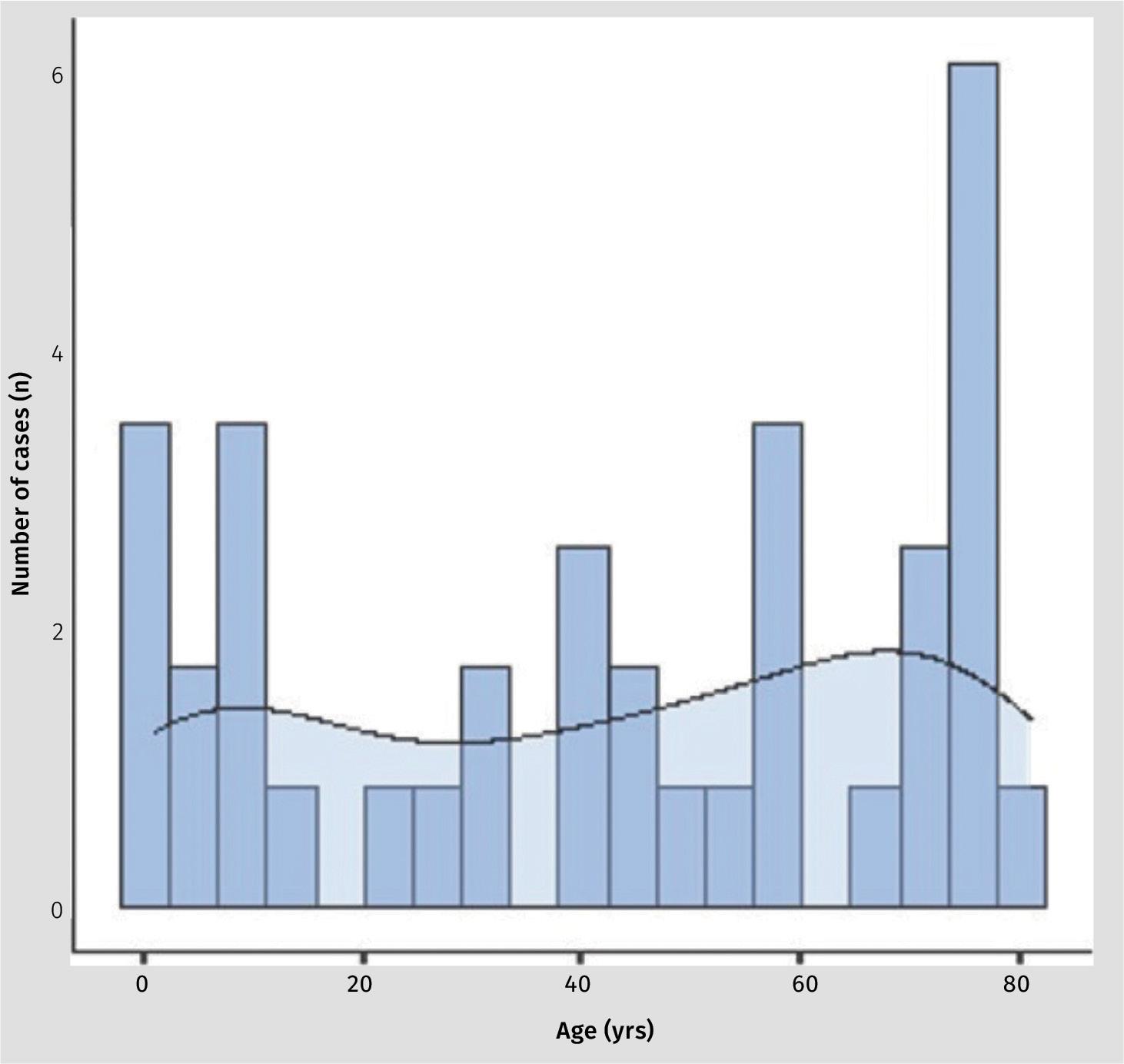
The mean age of patients was 39.4 years (SD 30.8; range 1.0 - 81.0). The spread between sexes was essentially equal with 14 (48%) being female and 15 (52%) being male. Figure 1 shows an even spread of cases over the age groups with a slightly
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 9 Original study Endophthalmitis microbial profile
Figure 1: Age distribution of cases.

denser preponderance of cases in the first and 8 th decade of life.
Most of our cases were from postsurgical endophthalmitis (n = 11; 39.9%), followed by post-traumatic endophthalmitis (n = 9; 31.0%), endogenous (n = 5; 17.2%) and post-intravitreal injection (n = 4; 13.8%) (Table III).
Overall microbiology yield was positive
in just over half of the samples (n = 16; 55.2%). No growth of any organism on culture was found in 13 (44.8%). The vast majority of the microbiological samples were from vitreous aspirates (n = 28; 97%) versus one aqueous aspirate (3%). Table II displays, in greater detail, the microbiology results on the positive culture yields.
The most common organism group cultured was Gram-positive cocci (n = 15; 83%). Within this Gram-positive group, staphylococci were predominant (n = 12; 80%) followed by streptococci (n = 2; 13.3%) and Enterococcus (n = 1; 6.7%). The most common staphylococcus organism was staphylococcus epidermidis (five cases) which was common across all mechanism categories. Gram-negative were far fewer with only three organisms cultured (17%). It is worth mentioning that two of the samples (from different patients) cultured two organisms each. One of these multiple-growth samples showed a mixture of Gram-positive (Streptococcus miti) and Gram-negative (Neisseria sicca).
When comparing culture-positive yields according to the mechanism of inoculation, endogenous cases had more culture positivity (80%) followed by postsurgical cases at 64%; post-intravitreal injection had a 50% positivity rate, while post-traumatic cases had the least positivity rate at 44% (Figure 2).
The most commonly cultured organisms by far across all mechanisms were Grampositive cocci (80%) and all Gram-positive organisms were either staphylococci or streptococci.
All 17 different microorganisms cultured were sensitive to the commonly tested antibiotics in our local NHLS laboratory, with only one Gram-positive isolate showing resistance to cloxacillin, erythromycin, and azithromycin. All Gramnegatives were sensitive to ceftazidime and all Gram-positives were sensitive to vancomycin. None of the cases in our

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 10 Original study Endophthalmitis microbial profile

study isolated fungal organisms despite being a subtropical coastal facility.
We had nine paediatric cases (28% of overall cases). The age range for this group was 1-9 years (median 5). The majority of these cases were post-surgical 4 (44%), three cases were post-traumatic (43%) and two were endogenous (22%). Of the total nine paediatric cases, only four were culture-positive (44%). Three cases showed Gram-positive cocci (two staphylococci and one streptococci). One case cultured a Gram-negative organism (Pseudomonas aeruginosa).
Discussion
The age spread of our cases showed a particular preponderance of paediatric and geriatric patients (see Figure 1). Most
of these cases were post-surgery. This is similar to findings in other similar studies.15,16 The cultured pathogenic organisms proved similar to those found in studies from other regions with predominant Gram-positive cocci with similar sensitivity profiles.1,6,17-23 The higher number of Staphylococcus epidermidis positive cultures could also be attributed to contamination with skin commensals, however, that is hard to determine as a matter of certainty by looking at the results as they are.
Our study revealed a preponderance of post-surgical and post-traumatic causes of endophthalmitis of which post-procedural surgical cases (either cataract surgery or intravitreal injections) were more common. This is similar to studies in high-income
countries like the United Kingdom and the United States of America 24 as well as in low to middle-income countries like China,1 Thailand, 25 and India.6,17
Interestingly, these results were different from research from a study in northern China where Liu et al. found a significant proportional predominance of post-traumatic endophthalmitis (49.6%) compared to post-surgical causes (26.7%). 20
Our positivity rates were comparable to other similar studies. Duan et al. also found a greater positivity yield in endogenous cases.1 However, what was slightly different in our study was that post-surgical cases tended to have a slightly better positivity rate than posttraumatic cases (Figure 2).
None of our cases were secondary to infective keratitis, unlike studies from other regions where they were often the majority.6,17,20,22,26 This is most likely attributable to our departmental protocol of not doing intraocular procedures (including diagnostic taps and antibiotic injections) in cases of infective keratitis, especially in the first three days after initiating topical antibiotic therapy.
Two out of our four culture-positive paediatric cases grew staphylococci. This result is dissimilar to that from a paediatric population study in China by Yang et al. 22 where they found more streptococci, especially in the lower-age paediatric groups.

Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 11 Original study Endophthalmitis microbial profile
Figure 2: Culture-positivity rates.
The current empiric antibiotic regimen we use in our local eye centres is intravitreal vancomycin to cover Grampositive organisms, and intravitreal ceftazidime to target Gram-negative bacilli and Pseudomonas. These guidelines are largely influenced by North American research. 7 These two antibiotics have been established as the standard empiric treatment in many eye centres around the world for many years, particularly since the Early Vitrectomy Study. 27
Some authors in other regions like India 28,29 and Australia 30 have demonstrated growing, albeit mild, resistance of pathogenic organisms to these two antibiotics. However, none of the cases in our study showed any resistance to either of these antibiotics in vitro.
One of our objectives was to determine whether this current empiric antimicrobial regimen is an appropriate first-line choice for all cases of bacterial endophthalmitis in our region. None of the cases in our study showed any resistance to a combination of Vancomycin and Ceftazidime. Furthermore, we have demonstrated no evidence of emerging resistance to these agents. Intravitreal vancomycin, with ceftazidime, therefore, continues to be an appropriate first-line treatment for bacterial endophthalmitis across all mechanisms of inoculation in our population. Table III shows our protocol specifics and preparation instructions.
Limitations
This was a retrospective study which inherently limits the strength of evidence.
We had a relatively small sample number. This is due to multiple factors, including the fact that endophthalmitis is a rare complication – most academic studies on this subject have similar or fewer numbers. 23,25,26,31 Also, our study was conducted at a quaternary level site that deals predominantly with the most difficult referrals, most of whom require pars plana vitrectomy, and therefore see fewer cases. We presume that cases in peripheral centres that were managed conservatively, with intravitreal antibiotics only, often did not make it to our study site.
Differences between in-vivo tests and clinical response to antibiotic treatment do exist. However, as outcomes were not assessed in this study, this could not be determined. Future studies could look at clinical outcomes with each variable measured (e.g. microbial spectrum measured against response to empiric treatment). To find out if these outcomes
are universal in our setting, it would be interesting to see future endophthalmitis studies from other eye units within the broader South African context.
Acknowledgements
Competing interests:
The authors have declared that no competing interest exist.
Authors’ contributions:
LGS prepared the protocol, collected the data and wrote the manuscript.
C-HK supervised the writing of the protocol, analysed the data and assisted with the preparation of the final manuscript.
Data availability statement:
The anonymised data underpinning this study will be shared upon reasonable request to the corresponding author.
Disclaimer:
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
No artificial intelligence-assisted technologies such as Large Language Models, chatbots, or image creators were used in the production of submitted work.
References
1. Duan F, Wu K, Liao J, Zheng Y, Yuan Z, Tan J, et al. Causative Microorganisms of Infectious Endophthalmitis: A 5-Year Retrospective Study. J Ophthalmol. 2016;2016:6764192.
2. Kernt M, Kampik A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clin Ophthalmol. 2010;4:121-35.
3. Wong TY, Chee S-P. The epidemiology of acute endophthalmitis after cataract surgery in an Asian population. Ophthalmology. 2004;111(4):699-705.
4. Chee S-P, Jap A. Endogenous endophthalmitis. Curr. Opin. Ophthalmol. 2001;12(6):464-70.
5. Krause L, Bechrakis NE, Heimann H, Kildal D, Foerster MH. Incidence and outcome of endophthalmitis over a 13-year period. Can J Ophthalmol. 2009;44(1):88-94.
6. Ramakrishnan R, Bharathi MJ, Shivkumar C, Mittal S, Meenakshi R, Khadeer MA, et al. Microbiological profile of culture-proven cases of exogenous and endogenous endophthalmitis: a 10-year retrospective study. Eye (Lond). 2009;23(4):945-56.
7. Han DP, Wisniewski SR, Wilson LA, Barza M, Vine AK, Doft BH, et al. Spectrum and susceptibilities of microbiologic isolates in
the Endophthalmitis Vitrectomy Study. Am J Ophthalmol. 1996;122(1):1-17.
8. Brillat-Zaratzian E, Bron A, Aptel F, Romanet JP, Cornut PL, Vandenesch F, et al. FRIENDS Group: clinical and microbiological characteristics of post-filtering surgery endophthalmitis. Graefes Arch Clin Exp Ophthalmol. 2014;252(1):101-7.
9. Gupta A, Gupta V, Gupta A, Dogra MR, Pandav SS, Ray P, et al. Spectrum and Clinical Profile of Post Cataract Surgery
Endophthalmitis in North India. Indian J. Ophthalmol. 2003;51(2):139-45.
10. Connell PP, O’Neill EC, Fabinyi D, Islam FMA, Buttery R, McCombe M, et al . Endogenous endophthalmitis: 10-year experience at a tertiary referral centre. Eye. 2011;25(1):66-72.
11. Mccannel CA. Meta-analysis of endophthalmitis after intravitreal injection of anti–vascular endothelial growth factor agents: Causative Organisms and Possible Prevention Strategies. Retina 2011;31(4):654-61.
12. Fintak DR, Shah GK, Blinder KJ, Regillo Cd, Pollack J, Heier JS, et al. Incidence of endophthalmitis related to intravitreal injection of bevacizumab and ranibizumab. Retina. 2008;28(10):1395-9.
13. Du Toit N, Mustak S, Cook C. Randomised controlled trial of prophylactic antibiotic treatment for the prevention of endophthalmitis after open globe injury at Groote Schuur Hospital. Br. J. Ophthalmol. 2017;101(7):862.
14. van der Merwe J, Mustak H, Cook C. Endophthalmitis prophylaxis with intracameral cefuroxime in South Africa. J Cataract Refract Surg . 2012;38(11):2054.
15. Relhan N, Forster RK, Flynn HW, Jr. Endophthalmitis: Then and Now. Am J Ophthalmol. 2018;187:xx-xxvii.
16. Peck TJ, Patel SN, Ho AC. Endophthalmitis after cataract surgery: an update on recent advances . Curr Opin Ophthalmol. 2021;32(1):62-8.
17. Bhattacharjee H, Bhattacharjee K, Gogoi K, Singh M, Singla BG, Yadav A. Microbial profile of the vitreous aspirates in culture proven exogenous endophthalmitis: A 10-year retrospective study. Indian J Med Microbiol . 2016;34(2):153-8.
18. Jindal A, Pathengay A, Mithal K, Jalali S, Mathai A, Pappuru RR, et al. Microbiologic spectrum and susceptibility of isolates in acute postcataract surgery endophthalmitis: are they same as they were more than a decade ago? Br J Ophthalmol 2014;98(3):414-6.
19. Kannan NB, Sen S, Mishra C, Lalitha P, Rameshkumar G, Rajan RP, et al. Comparative Study of Microbiological Profile and Management Outcomes of
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 12 Original study Endophthalmitis microbial profile
Acute Endophthalmitis after Microincision Vitrectomy Surgery versus Intravitreal Injections. Ocul Immunol Inflamm. 2021;29(5):838-44.
20. Liu Q, Wan L, Zhou J, Huang Y. TenYear Analysis of Pathogenic Factors and Etiological Characteristics of Endophthalmitis from a Tertiary Eye Center in North China. Infect Drug Resist. 2022;15:3005-12.
21. Lu LJ, Chen X, Adelman RA. Clinical Etiologies, Microbial Spectrum, Antibiotic Susceptibilities, and Visual Acuity Outcomes of Acute Endophthalmitis. J Ocul Pharmacol Ther. 2020;36(7):534-9.
22. Yang Y, Lin L, Li Y, Jiang Z, Li C, Liu M, et al. Etiology, microbiological isolates, and antibiotic susceptibilities in cultureproven pediatric endophthalmitis: a 9-year review. Graefes Arch Clin Exp Ophthalmol 2021;259(1):197-204.
23. Gupta A, Orlans HO, Hornby SJ, Bowler IC. Microbiology and visual outcomes of culture-positive bacterial endophthalmitis in Oxford, UK. Graefes Arch Clin Exp Ophthalmol. 2014;252(11):1825-30.
24. Miller JJ, Scott IU, Flynn HW, Jr., Smiddy WE, Newton J, Miller D. Acute-onset endophthalmitis after cataract surgery
(2000-2004): incidence, clinical settings, and visual acuity outcomes after treatment. Am J Ophthalmol. 2005;139(6):983-7.
25. Bhurayanontachai P, Klongthanakit P. A 14-Year Retrospective Analysis of Endogenous Endophthalmitis in a Tertiary Referral Center of Southern Thailand. J Ophthalmol. 2020;2020:6689081.
26. Sridhar J, Yonekawa Y, Kuriyan AE, Joseph A, Thomas BJ, Liang MC, et al. Microbiologic Spectrum and Visual Outcomes of AcuteOnset Endophthalmitis Undergoing Therapeutic Pars Plana Vitrectomy. Retina. 2017;37(7):1246-51.
27. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Arch Ophthalmol 1995;113(12):1479-96.
28. Shivaramaiah HS, Relhan N, Pathengay A, Mohan N, Flynn HW, Jr. Endophthalmitis caused by gram-positive bacteria resistant to vancomycin: Clinical settings, causative organisms, antimicrobial susceptibilities, and treatment outcomes. Am J Ophthalmol Case Rep. 2018;10:211-4.
29. Dave VP, Pathengay A, Nishant K, Pappuru


RR, Sharma S, Sharma P, et al. Clinical presentations, risk factors and outcomes of ceftazidime-resistant Gram-negative endophthalmitis. Clin Exp Ophthalmol. 2017;45(3):254-60.
30. Stevenson LJ, Dawkins RCH, Sheorey H, McGuinness MB, Hurley AH, Allen PJ. Gramnegative endophthalmitis: A prospective study examining the microbiology, clinical associations and visual outcomes following infection. Clin Exp Ophthalmol. 2020;48(6):813-20.
31. Malhotra S, Mandal P, Patanker G, Agrawal D. Clinical profile and visual outcome in cluster endophthalmitis following cataract surgery in Central India. Indian J Ophthalmol. 2008;56(2):157-8.
Copyright © 2023 Dr Lindelani G Shelembe. All rights reserved. Copyright subsists in the Author of this work. No part of this article or included photographs may be reproduced, published, performed, broadcast, transmitted or adapted in any form or by any electronic, mechanical or other means without the written permission of the copyright holder. This article is published by New Media, a division of Media24 (Pty) Ltd with consent of the Author. Any unauthorised reproduction, publishing, or adaption of this work will constitute copyright infringement and render the doer liable under both civil and criminal law.







Reduced propensity for elevation in intraocular pressure 6











An anti-infective and corticosteroid combination to treat a wide range of ocular infl ammation with infection or a risk of infection 1
0,5 % Loteprednol Etabonate with site-specific, high anti-inflammatory e cacy 2-4 0,3 % Tobramycin with broad spectrum activity 5






































Loteprednol etabonate 0.5% versus prednisolone acetate 1.0% for the treatment of inflammation after cataract surgery. J Cataract Refract Surg. 2013:39(2):168-73.

Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 13 Original study Endophthalmitis microbial profile
NEW
Br J Ophthalmol 2008;92:455– 459. 3. Dell SJ, et al. A randomized, double-masked, placebo-controlled parallel study of 0.2% loteprednol etabonate in patients with seasonal allergic conjunctivitis. J Allergy Clin Immunol 1998;102:251-5. 4. Gong L, et al. Loteprednol Etabonate Suspension 0.2% Administered QID Compared With Olopatadine Solution 0.1% Administered BID in the Treatment of Seasonal Allergic Conjunctivitis: A Multicenter, Randomized, Investigator Masked, Parallel Group Study in Chinese Patients. Clin Ther. 2012;34:1259–1272. 5. Comstock TL, Holland EJ. Loteprednol and tobramycin in combination: a review of their impact on current treatment regimens. Expert Opin Pharmacother. 2010 ;11(5):843-52. 6. Lane SS, Holland EJ.
Proprietary name and dosage form: Lotemax® Co Ophthalmic Suspension. Composition: Each 1 ml contains: Loteprednol etabonate 5,00 mg (0,5 % m/v), Tobramycin 3,00 mg (0,3 % m/v) and Benzalkonium chloride (preservative) 0,01 % m/v. Pharmacological classification: A 15.3 Ophthalmic preparations - combination antibiotics. Registration number 51/15.3/9038. For full prescribing information, refer to the professional information as approved by the South African Health Products Regulatory Authority (SAHPRA). © 2024 Bausch & Lomb Incorporated or its a liates. ®/TM denote trademarks of Bausch & Lomb Incorporated or its a liates. Soflens (Pty) Ltd. Reg. No.: 1968/011787/07. 254 Hall Street, Centurion, 0157. Tel: +27 10 025 2100. www.bausch.co.za. BL623/23
When inflammation and infection hits, strike back with our double-agent 1
Ocular relief to the rescue References: 1. Lotemax® Co package insert, April 2022. 2. Pavesio CE, et al. Treatment of ocular inflammatory conditions with loteprednol etabonate.
S4
The clinical profile of childhood blindness in a tertiary South African hospital - a 12-year review
Z Alashhab MBCHB, FICO, FC Ophth (SA), Registrar- Groote Schuur Hospital, Cape Town, South Africa.
ORCID: https//orcid.org/ 0009-0000-1600-2447
D Minnies PhD, MPH, NHDMedTech, NDMedTech, Director of the Community Eye Health institute - University of Cape Town, South Africa.
ORCID: https//orcid.org 0000-0002-9173-782X
C Tinley MBCHB, FRCOphth (London), Paediatric Ophthalmologist - University of Cape Town and Atlantic Eye Centre, Cape Town, South Africa.
ORCID: https//orcid .org 0000-0001-5817-7122
Corresponding author: Dr Zakaria Alashhab. e-mail: zakariaalashhab@gmail.com
Abstract
Aims: To investigate the causes of blindness among children aged 0-13 years in Cape Town over a 12-year period.
Methods: A retrospective review was conducted of all children registered blind between 2011-2022 at the Red Cross War Memorial Children’s Hospital. The children were diagnosed as blind according to the WHO criteria and referred to local blind schools. Data on anatomical site, aetiology and avoidable causes were collected using the WHO/PBL Eye Examination Record. Descriptive statistics were used for analysis.
Results: A total of 182 blind children were identified. The most common anatomical sites of blindness were the optic nerve (27%), retina (23.6%) and normal globe (21.4%), followed by whole globe (18.7%), uvea (3.3%), lens (3.3%), and cornea (2.7%). Regarding aetiological causes, hereditary conditions were responsible for (23.5%), perinatal factors (15.3%), postnatal factors (9.3%), intrauterine factors (3.3%), but the largest proportion of cases (48.5%), fell under disorders of
Introduction
Childhood blindness has been a priority area for the Vision 2020: The Right to Sight initiative of the World Health Organization (WHO), launched in 1999.1
According to estimates at that time, there were globally 1.4 million blind children, with twice this number having low vision, and approximately 500,000 new cases of childhood blindness occurring each year.1
Childhood blindness is important not only because these children face a lifetime of vision impairment, but also because it carries profoundly negative psychological, educational, and economic impacts for the affected individuals as well as their
unknown aetiology. Avoidable causes accounted for (22.5%) of cases, and the majority of these were cerebral palsy/neonatal asphyxia (7.1%).
Conclusions: We found optic nerve (27%), retina (23.6%) and normal globe (21.4% - mainly cerebral visual impairment) to be the most common causes of childhood blindness in Cape Town. These proportions are more closely aligned with developed nations and indicate a different clinical profile when compared to prior, nationwide studies. This information may help guide future public health strategies to target the specific causes identified.
Keywords: Childhood blindness, visual impairment, pediatric ophthalmology, Cape Town, South Africa.
Conflict of interest : None of the authors have conflicts of interest related to this submission.
communities. 2 The causes of blindness in children have been linked to higher rates of child mortality. 3 Furthermore, many causes of childhood blindness are either preventable or treatable. 3
The distribution of causes of childhood blindness differs markedly between regions according to socioeconomic factors. In the lowest-income countries, corneal scarring leading to vision impairment is most prevalent, whereas high-income nations report lesions of the central nervous system as being predominant. Middle-income countries present a mixed epidemiological picture. Retinopathy of prematurity
has increasingly become an important avoidable cause of blindness in this setting, as survival rates of preterm infants have risen. Additionally, across all regions, cataract, retinal diseases, and congenital anomalies affecting the whole globe constitute significant contributors to childhood visual impairment. 3,4 Data from one country can therefore not be extrapolated to another. 5 Accurate data on the causes of blindness is needed for appropriate allocation of resources towards preventive and curative services.6 Some of the causes may require primary level public health interventions, such as immunisation and nutrition to
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 14 Original study Profile of childhood blindness
prevent corneal scarring, whereas others need tertiary-level intervention, such as surgery and low-vision services for cataract, retinopathy of prematurity and glaucoma. 7 Data on childhood blindness in South Africa is sparse. The most recent study looked at causes of blindness in a Johannesburg blind school and the other was a broader, cross-sectional survey conducted almost thirty years ago. 8,9
The aim of this study was to provide current data on the clinical profile of childhood blindness in the Western Cape province of South Africa and compare the pattern of childhood blindness with local and international published literature. Hopefully, this will assist local authorities in planning appropriate strategies to implement preventive, curative, and rehabilitative services.
Materials and methods
We reviewed the causes of childhood blindness over a 12-year period in the ophthalmology department of the Red Cross War Memorial Children’s Hospital in Cape Town, South Africa. The hospital is one of two large tertiary referral centers serving Cape Town and its surrounding areas. UNICEF defines a child as an individual aged less than 16 years; at this institution, children are treated until age 13, with limited adolescent services. The WHO International Classification of Diseases-10 definition of visual impairment was utilised.10 This classification defines blindness as a best corrected visual acuity of less than 3/60 or a visual field less than 10 degrees in the better-seeing eye.
We performed a retrospective folder review of all children diagnosed with visual impairment, who were referred to three blind schools in Cape Town, from January 2011 to December 2022. The primary source of information was the hospital referral forms to the local blind schools, in the minority of cases, where data on the referral forms were insufficient, clinical records were retrieved to investigate findings. All causes of blindness were recorded according to the WHO/PBL Eye Examination Record for Children with Blindness and Low Vision Instructions.11 This provides definitions and methods of classification for anatomical, aetiological and avoidable causes of visual loss. The anatomical classification marks the major site of abnormality leading to blindness. These include whole globe, cornea, lens, uvea, retina, optic nerve, and globe appears normal. The aetiological classification refers to the timing of the
insult leading to visual loss. These include hereditary disease, intrauterine, perinatal/ neonatal factors, postnatal/infancy/ childhood factors and cannot determine/ unknown aetiology. Avoidable causes were also determined, either preventable or treatable. After examination, the major site of abnormality and aetiology were determined for each eye and individual. Ethical and institutional approvals were obtained from the University of Cape Town Human Research Ethics Committee and the Red Cross War Memorial Children’s Hospital. Descriptive statistical analyses were used and presented as frequencies and percentages.
Results
Between 2011 to 2022, 182 children were diagnosed as being blind according to WHO criteria. 96 (52.7%) were male, while 86 (47.3%) were female. The mean age of the children was 3.8 years, with a standard deviation of 3.2 years. And the median age was 2.69 years, with interquartile range 1.34-6.01.
Anatomical site of visual abnormality
The anatomical sites of abnormality in the children are shown in Table I. Optic nerve, retinal conditions, and ‘the globe appears normal’ were the three most common causes. Optic nerve conditions were the most common site of visual impairment, with 49 children affected (27%). These included optic nerve atrophy due to hydrocephalus, which impacted 20 children (11%) and optic nerve hypoplasia, affecting 16 children (8.4%). Retinal conditions were the second most common cause, present in 43 children (23.6%). Of these, 11 children (5.8%) had oculocutaneous albinism (OCA). Fourteen children had retinal dystrophies including Leber’s congenital amaurosis, retinitis pigmentosa, and BardetBiedl syndrome, while ROP and retinal detachment found in five (2.7%) and three (1.6%) children, respectively. One child had a history of bilateral retinoblastoma. Thirty-nine children (21.4%) had normal ocular examinations and most of these cases were attributed to cerebral visual impairment. Abnormalities of the whole globe were seen in 34 children (18.7%). Microphthalmia was the leading cause, present in 16 children (8.4%). Glaucoma was present in five children (2.7%) and corneal scarring found in five children (2.7%), with causes identified as Peter’s anomaly and other congenital corneal opacities. Uveal conditions were found
in six children (3.3%); five had aniridia and one had bilateral chorioretinal colobomas. Cataract was the cause of visual impairment in six children (3.3%), mainly due to late diagnosis and severe amblyopia after cataract surgery.

Aetiology of visual loss
Hereditary diseases accounted for 23.6% of cases. Most of these conditions were oculocutaneous albinism and retinal dystrophies. Intrauterine factors such as congenital rubella syndrome and cytomegalovirus infection contributed to a smaller percentage, of 3.3%.
Perinatal factors, which included cerebral palsy and retinopathy of prematurity, resulted in visual impairment in 15.3% of children. Postnatal childhood infections and trauma resulted in 9.3% of cases. However, the largest proportion of cases, 48.5%, fell under disorders of unknown aetiology. These included conditions such as hydrocephalus, microphthalmos, anophthalmos, glaucoma and optic nerve hypoplasia, which could not be clearly attributed to hereditary, intrauterine, or perinatal risk factors. (Table II).

Avoidable causes
The term ‘avoidable blindness’ is used to encompass the conditions causing blindness which can be prevented or treated. Avoidable causes of visual impairment are shown in Table III. Fortyone children (22.5%) had underlying causes that could have been avoided. These comprised 25 (13.7%) with conditions amenable to primary prevention, such
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 15 Original study Profile of childhood blindness
as cerebral palsy, neonatal infections, and trauma, as well as 16 (8.8%) with visual impairment secondary to treatable conditions, including cataract, glaucoma, and retinopathy of prematurity.

Discussion
The WHO has reported a notable variation in childhood blindness prevalence according to socioeconomic development levels across regions. Specifically, they found higher rates of visual impairment in lower-income areas, and the estimated global prevalence of blindness among children was approximately 0.75/1000 children in 1999.12 This study contributes novel insights into the causes of childhood blindness among children in Cape Town. O’Sullivan et al. provided a comprehensive overview of childhood blindness across South Africa in 1997 and in 2020, Esra reported on visual impairment in children in a school for the blind in Johannesburg. 8,9
These studies found different leading causes of childhood blindness when compared to ours. Esra reported retinal conditions as the primary cause of blindness in school children in 42% of cases, followed by whole globe abnormalities in 16% of cases. Similarly, O’Sullivan found retina to account for 38.5% of childhood blindness, with optic nerve being the second highest at 15.2%. 8,9
The high prevalence of retinal disorders in these earlier studies was attributed to high rates of retinopathy of prematurity, aligning them more closely with developing country profiles. Additionally, the O’Sullivan et al. study identified cornea as a major cause (11.2%), consistent with typical patterns across sub-Saharan Africa. (Table IV)
In Cape Town, the top three anatomical
sites contributing to blindness were the optic nerve, retina, and cases where the globe appeared normal. This clinical profile is more closely aligned to that of childhood blindness in the developed nations. When compared to other provinces in South Africa, the Western Cape is known to perform better in terms of health care delivery. It scored amongst the highest of several clinical, management and financial indicators, including provincial expenditure on the district health system in 2011-12 (DHB 20112012). In the 2019-2020 financial year, the Western Cape recorded the second highest proportion of “Ideal Clinics” (a measure of adherence to high quality performance) and “Core essential medicines availability”, after Gauteng, the province with the highest gross domestic product in Africa. (https://www.gov.za/provinces). The Western Cape Province also reported the highest ratio of medical practitioners per 100 000 population in the public sector in the same period. (DHB 2019-2020). Hence the clinical profile of childhood blindness found here may not be generalisable to the rest of the country, or indeed the larger African continent.
The leading anatomical site of childhood blindness found in this study was optic nerve disease in 27% of cases. This proportion is significantly higher than reported in previous South African studies. The Johannesburg school for the blind study recorded optic nerve disease in just 10% of children, while the O’Sullivan et al. study reported it at 15.2%. 8,9 Our figure of 27% is more aligned with data from established market economy (EME) countries,12 where optic nerve conditions have been attributed to approximately 25% of visual impairment. (Table IV ) Retinal pathology also represented a significant proportion of visual impairment cases in our study (23.6%). It included two prominent aetiologies - retinal dystrophies
and oculocutaneous albinism, accounting for 8.2% and 5.6% of cases respectively. The third highest cause was where the globe appeared normal (21.4%), the majority of these cases were attributed to cerebral visual impairment (20.3%), these data match closely to data on the anatomical causes of blindness for EME countries.
Congenital anomalies affecting the whole globe were also common, affecting 15.3% of cases. Genetic diseases and intrauterine factors are the likely underlying reasons for these conditions, although the causes remain unknown in most cases. There seems to be large regional differences in the percentages of blind school children with congenital anomalies. Data shows the rates ranging from 1.4% of blind students in Cuba to as high as 33.2% in Sri Lanka.13 This wide range suggests that factors that influence the prevalence of such anomalies may vary significantly between locations.
Glaucoma accounted for 2.7% of treatable visual loss cases in this study. This was lower than the value documented in the previous 1997 national study of childhood blindness in South Africa by O’Sullivan et al., which found glaucoma in 6.7% of cases. 8 This study’s lower percentage is more similar to studies from developed world regions, such as 1.1% of cases in New Zealand and 5% of cases in the UK.14,15 The glaucoma prevalence observed here was also lower than values documented for other African countries. For example, glaucoma caused blindness in approximately 9% of pediatric populations in both Ghana and Nigeria.16,17 Our findings therefore indicate the glaucoma burden in Cape Town children appears more closely aligned to developed nations than other parts of the continent.
Congenital cataract is a leading cause of surgically correctable blindness worldwide, with a reported global incidence ranging from one to 15 per

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 16 Original study
of childhood blindness
Profile
10,000 live births.18 However, data on childhood cataract prevalence specifically in South Africa is limited. In this study, lens-related disorders accounted for 3.3% of visual impairment cases, which is lower than the 3.7% reported in the previous 1997 national study of childhood blindness in South Africa by O’Sullivan et al. and is less than percentages reported for EME countries (8%) and globally (12%). This study’s low rate approximates the 3.6% reported in New Zealand. 12,14
ROP was an unexpectedly uncommon finding in our study, with only five identified cases accounting for 2.7% of all childhood blindness causes. This is significantly lower than what was found in the previous study from 25 years ago, which reported ROP accounting for 10.6% of childhood blindness cases. 8 However, the epidemiology of ROP-induced blindness is changing. In developed nations, implemented screening protocols have proven highly effective at reducing ROP rates.19 But it is emerging as an increasingly important, yet avoidable, cause of vision impairment in middleincome countries. This shift reflects improvements in perinatal care that have raised survival rates of preterm infants who are most at risk of developing ROP.19
In the developing world, the main pathology leading to blindness is corneal scarring resulting from conditions such as vitamin A deficiency, measles infection, ophthalmia neonatorum, or harmful traditional remedies. 20 However, there were no such cases recorded in this study. This result is much improved compared to 25 years ago in South Africa, when corneal scarring accounted for 11.2% of childhood blindness cases. 8
A significant proportion of our cases, (22.5%) fell under the WHO classification of avoidable causes. Previous research suggests approximately two-thirds of childhood blindness in low-income countries is avoidable. 21 We found 22.5% of causes were avoidable, through either prevention or treatment. The reduction in blindness due to avoidable causes in South Africa, from 38.8% in previous local study to the current 22.5%, reflects the successful implementation of large-scale primary healthcare initiatives. 8 Campaigns have facilitated wide distribution of measles and rubella immunisation through South Africa’s Expanded Program on Immunisation (EPI-SA), initiated in 1995. Additionally, programmes have supported newborn eye prophylaxis and vitamin A supplementation. 22
While eradicating all preventable causes of blindness remains a priority, it is also apparent that confronting unavoidable conditions like microphthalmos, congenital exophthalmos and retinal dystrophies poses a significant, ongoing challenge. Further research is needed to better understand the genetic and pathological underpinnings of these disorders. Advances in technologies such as genetic testing and counseling may help reduce the incidence of blindness due to inherited diseases.
Limitations
The findings of this study relate to a cohort of children diagnosed at a tertiary eye care facility in an urban setting in South Africa and may not necessarily be generalisable to the country as a whole. Some limitations existed when reporting visual impairment rates from blind school application forms. However, missing data was minimised by retrieving patient clinical notes wherever the referral forms were incomplete. We only included children from birth to 13 years in our study and did not report on those aged 13 to 16, who are still defined as children under the UNICEF criteria.
Conclusion
We found optic nerve (27%), retina (23.5%) and normal globe (21.4% - mainly cerebral visual impairment) to be the most common causes of childhood blindness in Cape Town during the 12-year study period. These proportions are more closely aligned with the developed nations and indicate a different clinical profile when compared to prior, nationwide studies. This information may help guide future public health strategies to target the specific causes identified.
References
1. World Health Organization. Global initiative for the elimination of avoidable blindness. WHO/PBL/97.61 Rev.2.
2. Gilbert CE, Anderton L, Dandona L, et al. Prevalence of visual impairment in children: a review of available data. Ophthalmic epidemiol 1999:6:73-82.
3. Gilbert CE. New issues in childhood blindness. Community eye health. 2001:15:53-56.
4. Gilbert CE, R ahi J, Quinn G. Visual impairment and blindness in children. The epidemiology of eye disease. 2003:260-286.
5. Foster A. Childhood blindness. Eye. 1988:2:27-36.
6. Rahi JS, Sripathi S, Gilbert CE, et al. Childhood blindness in India: causes in 1318 blind school
students in nine states. Eye. 1995:9:545-50.
7. Gyawali R, Moodley VR. Causes of Childhood Vision Impairment in the School for the Blind in Eritrea. Optom.Vis. Sci. 2017:94:1138-1144.
8. O’ Sullivan J, Gilbert C, Foster A. The causes of childhood blindness in South Africa. S. Afr. Med. J. 1997:87:1691-1695.
9. Esra N, Mayet I. The causes of visual impairment in children in a school for the blind in Johannesburg. S. Afr. Med. J. 2020:15:26-29.
10. World Health Organization. Manual of the International Statistical Classification of Diseases, Injuries, and Causes of Death. Geneva: 1977.
11. Gilbert C, Foster A, Negrel AD, Thylefors B. Childhood blindness: a new form for recording causes of visual loss in children. Bull World Health Organ 1993; 71(5): 485–489.
12. World Health Organization. Preventing blindness in children. Hyderabad, India. 1999. Contract No.: WHO/PBL/00.77
13. Eckstein MB, Foster A, Gilbert C. Causes of childhood blindness in Sri Lanka. Br J Ophthalmol 1995; 79: 633–636.
14. CheeFoong C, Charles N, Shuan H. Causes of childhood low vision and blindness in New Zealand. Clin Exp Ophthalmol 2019:47:165-170
15. Lucinda J Teoh, Ameenat Lola Solebo, Jugnoo S Rahi, on behalf of the British Childhood Visual Impairment and Blindness Study
Interest Group. Visual impairment, severe visual impairment, and blindness in children in Britain (BCVIS2): a national observational study. Lancet Child Adolesc Health 2021; 5:190–200.
16. Grace J, Judith S, S Grace P. Causes of childhood blindness in Ghana: results from a blind school survey in Upper West Region, Ghana, and review of the literature. Int. Ophthalmol. 2018:38:1415-1423.
17. I R Ezegwui, R E Umeh, U F Ezepue. Causes of childhood blindness: results from schools for the blind in southeastern Nigeria. Br J Ophthalmol 2003;87:20–23.
18. Gilbert C, Rahi J, Quinn G. Visual impairment and blindness in children. In: Johnson G, Minassian D, Weale W, West S (eds). Epidemiology of Eye Disease 2003 2nd ed:260–286.
19. Gilbert C, Rahi J, Eckstein M, O’Sullivan J, Foster A. Retinopathy of prematurity in middle income countries . Lancet 1997; 350: 12–14.
20. Gilbert C, Foster A. Childhood blindness in the context of VISION 2020–The right to Sight. Bull World Health Organ 2001; 79: 227–232.
21. World Health Organization. Report of WHO/ IAPB Scientific Meeting. World Health Organization; 2000.
22. Baker L. The face of South Africa’s Expanded Program on Immunisation (EPI) schedule. S Afr Pharm J. 2010;77(1):18-49.
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 17 Original study Profile of childhood blindness
TREAT to keep more light than TUNNEL 1,2
The only3 fixed combination TCAI/AA*1
Decrease of elevated intraocular pressure (IOP) in adult patients with open-angle glaucoma or ocular hypertension for whom monotherapy provides insufficient IOP reduction.1

SIMBRINZA®. Enabling MMT*† when added to PGA/ timolol combination therapy.4
• achieves maximal intraocular pressure (IOP) reduction4
• minimises adverse effects4
• addresses compliance challenges4
* The use of ≥3 classes of topical antiglaucoma agents
PGA = Prostaglandin analogues; †MMT = Maximal medical therapy; ≠AA = α2-adrenergic agonists; TCAI = topical carbonic anhydrase inhibitors

Scan
twice daily 1
DROP
TEST • TREAT • PRESERVE sight 1
QR code to view approved Professional Information References: 1. Simbrinza Professional Information. Novartis (Pty) Ltd. 06 February 2023. 2. Rumelt S, Schreiber S. Why Do Patients with Controlled Glaucoma Continue to Lose Their Vision?. In: Rumelt S, editor. Causes and Coping with Visual Impairment and Blindness [Internet]. London: IntechOpen; 2018 [cited 2022 May 17]. Available from: https://www.intechopen.com/chapters/63311
IQVIA MAT Mar 2023 (ATC: S1E). 4. Lerner SF, Oddone F, Lu D-W, et al Maximum
brimonidine
travoprost/timolol
in glaucoma
ocular hypertension.
13
2411–2419.
full prescribing information,
the
the South African Health Products Regulatory Authority (SAHPRA). S3 SIMBRINZA® 10 mg/ml + 2 mg/ml eye drops, suspension. Reg. No.: 50/15.4/0358. Each 1 ml of suspension contains 10 mg of brinzolamide and 2 mg of brimonidine tartrate. Holder of Certificate of Registration: Novartis South Africa (Pty) Ltd. Magwa Crescent West, Waterfall City, Jukskei View 2090. Tel. +27 11 347 6600. Co. Reg. No. 1946/020671/07. Novartis Adverse Drug Reaction Reporting: Email: patientsafety.sacg@novartis.com. Web: https://www.report.novartis.com/. Tel: 0861 929-929. Marketed and Distributed by Adcock Ingram Limited. Co. Reg. No. 1949/034385/06. Private Bag X69, Bryanston, 2021. Customer Care: 0860 ADCOCK / 232625. www.adcock.com ZA2305265947 Exp Date 05/2025 45207 06/23
doi: 10.5772/intechopen.797. 3.
medical therapy: Brinzolamide/
and
fixed-dose combinations
and
Clinical Ophthalmology 2019;
:
For
refer to
Professional Information Approved by

The utility of a non-mydriatic fundus camera in a tertiary diabetes clinic
S Ben Barka, MBChB, FCP (SA), MMed (Int), Cert Endo and Metab (SA), Fellow, Division of Endocrinology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0009-0000-6904-574X
C Laurence, MBChB MSc, Clinical Epidemiology Medical Officer, Division of Endocrinology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0002-9250-6193
L Du Toit-De Wet, MBChB, FC Ophth, MMED (Opt), Dip Ophth (SA), Consultant, Division of Ophthalmology, Department of Surgery, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0001-55 67-1109
M Conradie-Smit, MBChB, MMed (Int), FCP (SA), Cert Endo and Metab (SA), Consultant, Division of Endocrinology, Department of Medicine, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0002-4252-6647
Corresponding author: Samia Ben Barka, email: ssbenbarka.bbss@gmail.com
Abstract
Background: Diabetes mellitus (DM) is rapidly escalating in prevalence globally. This is affecting health systems everywhere, especially in developing countries where these are already overburdened. Diabetes is the leading cause of blindness in the world and early detection of retinopathy is paramount in preventing unwanted sequelae.
Aim: The study aimed to assess the utility of a non-mydriatic fundus camera to screen for eye disease in the Autoimmune Diabetes Mellitus (DM) clinic (Endocrinology Division) of Tygerberg Hospital (TBH), in Cape Town, South Africa.
Our primary objective was to quantify what proportion of patients screened had severe eye disease requiring urgent referral to the Ophthalmology service at the same institution.
The secondary objective was to quantify the proportion of patients with mild or no disease that were not referred to the eye clinic and who were suitable to be followed up in the DM clinic by a trained medical officer.
Method: This was a retrospective descriptive study. Ethical approval was obtained from the Health Research Ethics Committee (Ethics Reference Number S21/08/156) of Stellenbosch University. In addition, institutional approval was obtained (WC_202206_020).
The study population included patients with Type 1 DM, or other auto-immune DM, who underwent digital photographic retinal screening during a six-month period from Sept 2019 to March 2020.
Results: A total of 177 patients were included in the study. The majority had Type 1 DM (n = 153, 86.4%) with latent autoimmune diabetes mellitus (LADA) accounting for the rest (n = 24, 13.6%). Severe diabetic retinopathy (DR) was detected in 7.9% of patients (n = 14), resulting in 2.8% (n = 5) requiring urgent referral to Ophthalmology and the rest semi-urgent. Normal screening or only mild disease were detected in 78% (n = 138). This resulted in 78.5% (n = 139) of patients not requiring referral
to the Ophthalmology service. The remaining 17.7% (n = 31) were referred routinely.
Conclusion: The use of a non-mydriatic fundus camera to detect eye disease is known to be highly effective as a classifying/ evaluating tool of the severity of Diabetes Retinopathy. In our study it assisted with appropriate urgent referral to Ophthalmology services in patients with severe diabetic retinopathy. It also allows for not to refer mild retinopathy disease to Ophthalmology clinic, thereby preventing unnecessary hospital visits for patients. Consequently, the tertiary level diabetes clinic’s screening approach demonstrated an excellent tool for prioritising high-risk patients so they could receive appropriate care without delay.
Keywords: Diabetes mellitus, retinopathy, non-mydriatic fundus camera, screening, ophthalmology referral.
Funding: Nil received.
Conflict of interest: None.
Ethics approval, including consent: HREC no (S21/08/156) WCG no (WC_202206_020).
The research was done as part of an MPhil degree, with successful submission of the thesis and the degree to be awarded end 2023.
List of abbreviations
DM Diabetes mellitus
HIV Human Immunodeficiency Virus
LADA Latent Autoimmune Diabetes in Adults
WHO Word Health Organization
HbA1c Haemoglobin A1c (Glycated haemoglobin)
GDM Gestational Diabetes mellitus
TBH Tygerberg Hospital
PLWD People living with diabetes
NMFC Non-mydriatic fundus camera
DED Diabetes eye disease
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 19 Original study Non-mydriatic fundus
Introduction
Diabetes mellitus (DM) is a metabolic disease/ malady characterised by hyperglycaemia, with rapidly escalating prevalence globally.1 This is affecting health systems worldwide, especially in developing countries. 2,3 In Africa specifically where health systems are already overburdened by communicable diseases such as HIV, tuberculosis and malaria, DM prevalence will double from 12 million in 2010 to 24 million by 2030.4 In 2019, a total of 463 million people worldwide were estimated to be living with DM, representing 9.3% of the global adult population (20-79 years of age). This number is expected to increase to 700 million (10.9%) in 2045.1 As DM prevalence increases, prevalence of DM complications will increase.
DM is one of the major noncommunicable diseases (NCD) causing death. 5,6 NCD include heart disease, stroke, cancer, chronic lung disease and DM, and these are collectively responsible for almost 70% of all deaths worldwide. Concerningly, 77% of all NCD deaths are in low- and middle-income countries, of which South Africa is one.7
DM causes macrovascular and microvascular (diabetic eye disease (DED), diabetic kidney disease and neuropathy) complications. DED is a group of ocular conditions that may occur in patients with DM. These conditions include, but are not limited to, cataracts at a younger age, diabetic retinopathy (DR), diabetic macular oedema (DME) and glaucoma.8 The complications of DM, including retinopathy, are correlated with the duration of DM, poor glycaemic control (high level of glycated haemoglobin [HbA1c]),9 and the presence of other vascular risk factors such as dyslipidemia10 and high blood pressure.11,12 Therefore, the importance of early detection of microvascular complications is paramount in mitigating risk, both by stricter glycaemic control and by optimising the management of other risk factors. The presence of microvascular complications is associated with macrovascular complications and cardiovascular risk.13,14,15 With advanced retinopathy the risk is increased for ischemic heart disease (IHD), as well as chronic kidney disease (CKD), stroke and dementia.16,17
There are several barriers impeding the early detection of retinopathy in resource limited settings.18 These include a lack of screening programmes in certain areas, lack of education of both patients and health care workers at the primary care level regarding the importance of DR, financial barriers and barriers in access to
eye care such as rural settings.19 The use of dilating eye drops prior to fundoscopy is an added limitation due to the inconvenience of cycloplegia preventing accommodation and hence affecting the ability to read, drive and return to work for 4 to 10 hours.18,20 The process may also be timeconsuming and requires special skills.
As the prevalence of DM increases, the burden of screening for the complications, especially DED, becomes more challenging. 21 Modern techniques for screening for diabetic eye disease have improved significantly in the last few years, becoming more practical and more accurate. 22 This is achieved by using technology such as non-mydriatic fundus photography and artificial intelligence software to detect pathology. 23,22 In addition, systems have been developed to grade DR as well as to possibly detect other pathology such as hypertensive retinopathy or cataract. 24,25 Screening effectively detects the early development of DED and enables timeous treatment of eye disease, 26 also in our setting. 27
The study aimed to assess the utility of a NMFC (non-mydriatic fundus camera) to screen for DED in patients from the autoimmune DM clinic of TBH, a tertiary level facility in the Western Cape province of South Africa. Our primary objective was to quantify what proportion of screened patients had severe DED requiring urgent referral to the Ophthalmology service. The secondary objective was to quantify the proportion of patients with mild or no disease who were not referred to the eye clinic and who could therefore undergo further retinal screening at the DM clinic.
Materials and methods
This was a retrospective descriptive study where patients were selected using consecutive sampling. Ethical approval was obtained from the Health Research Ethics Committee of Stellenbosch University (Ethics Reference Number S21/08/156). In addition, institutional approval was obtained from Tygerberg hospital (WC_202206_020).
We included all patients who attended the auto-immune DM clinic (new and followup patients) and had retinal photographic screening during a six-month period from Sept 2019 to March 2020. This DM clinic was attended by adult and adolescent patients as it is dependent on the age of diagnosis in certain cases. We excluded patients with Type 2 DM, ketosis prone Type 2 DM and pregnant patients, should they have been screened. The reason was that we wanted to describe the impact of this intervention
in the auto-immune DM population, prior to expansion to other cohorts.
We used a Canon Digital Retinal Camera CR-2 AF (CANON INC 30-2, SHIMOMARUKO 3-CHOME, OHTA-KU, TOKYO, JAPAN) to photograph the full fundus of each eye without dilating the pupil. Photos were repeated until a good quality photo was obtained. All photographic screening was performed by one endocrinology medical officer who was trained by staff from the ophthalmology service to do screening and worked in a darkened room adjacent to the DM clinic. The images were then reviewed by a doctor from the Ophthalmology clinic on the day. We used the Early Treatment Diabetic Retinopathy Study (ETDRS) classification to grade retinopathy. 28
Data were collected from the database on the camera as well as medical records where applicable and recorded using a Microsoft Excel spreadsheet. Data included were age of the patient in years, sex of the patient, type of DM (type one or Latent Autoimmune Diabetes of Adulthood), duration of DM in years, weight (kilograms), HbA1c (%) and the presence of other cardiovascular risk factors (dyslipidaemia, smoking and blood pressure), other documented complications of diabetes (microvascular/ macrovascular) and applicable socioeconomic information such as employment. The privacy of personal information was ensured by using coded identities and the data were stored on a single computer with password protection.
The Division of Biostatistics and Epidemiology of the University of Stellenbosch assisted with the statistical analysis of the data. Stata version 16 was used to analyse the data. Descriptive statistics were utilised to estimate the proportions of interest in the study population such as the proportion of patients referred on an urgent basis due to severe retinopathy (primary outcome) and the proportion of patients who continued screening at the DM clinic (secondary outcome). The proportion of the latter group of patients who were employed and who did not have to attend a second time for screening in the ophthalmology department, was also calculated.
Results
A total of 180 patients were screened over the six months period at the DM clinic. Three patients were excluded as they had Type 2 DM. The majority of patients were female (n = 107, 60.5%). The mean age was 30.2 ± 9.7 years (range 15-62 years). Most patients were known with Type 1
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 20 Original study Non-mydriatic fundus
DM (n = 153, 86.4%) and the rest had LADA (n = 24, 13.6%), Mean DM duration was 12.3 ± 8.3 years. A total of 175 patients were using insulin with a mean total daily insulin dose of 54.0 ± 23.7 units. Mean HbA1c was 10.5 ± 2.5% (range 4.9 to 18.4%).
Baseline characteristics are shown in (Table I).

Other cardiovascular risk factors were documented. Dyslipidaemia was most common in 77 patients (44.3%), followed by smoking (57 patients, 32.85%) and hypertension (44 patients,25.1%). (Table II)

We documented the presence of other microvascular complications. For diabetic kidney disease, 95 patients (53.7%) were disease-free, while 76 patients (46.3%) were affected. The majority had
severely increased albuminuria (more than (>300mg/day) (n = 54,30.5%), with moderately increased albuminuria in 11 patients (6.2%). A decreased glomerular filtration rate (GFR) was detected in 13 patients (7.3%). Four patients were not tested for kidney disease.
Peripheral and autonomic neuropathy was only detected in 27 (15.3%) and 12 (6%) patients respectively. (Table III)

Diabetic retinopathy was not detected in 138 patients (78.0%), mild retinopathy occurred in 14 (8.0%), moderate in five (2.8%) and severe in 14 (8.0%) of patients. Maculopathy was detected in four patients (2.3%). In two patients (1.3%), evaluation was not possible due to the presence of cataracts. (Figure 1).
Therefore, a total of 139 patients (78.5%)
were not referred for ophthalmology review. Furthermore, five patients (2.86%) were referred as priority cases for urgent ophthalmology review and 31 patients (17.71%) were referred for routine evaluation. (Table IV ).

Employment status was only recorded in 102 patients (57.6% of total). The majority of these patients were unemployed (60 patients). In the case of the employed patients, 34 (80.9%) were not referred to ophthalmology thereby avoiding attendance on another day and missing an additional day of work.
Discussion
This retrospective descriptive study evaluated the utility of NMFC in a tertiary auto-immune diabetes clinic in detecting diabetic retinopathy. It was noted in our clinic that patients who are followed in a tertiary setting do not present to these primary level screening programmes. Prior to this study these patients were all referred by our clinic to the Ophthalmology service on a regular basis for screening and follow-up.
In our study population, diabetic retinopathy was not detected in 138 patients (78.0%), mild retinopathy occurred in 14 (8.0%), moderate in five (2.8%) and severe in 14 (8.0%) of patients. This had two main advantages: firstly, patients with significant disease were detected earlier with urgent referral to the Ophthalmology

Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 21 Original study Non-mydriatic fundus
Figure 1: Fundus camera screen result.
service for timeous intervention and, secondly, patients with normal screening results or mild disease, which accounted for almost 80% of patients screened, could be followed up in the DM clinic, without unnecessarily overburdening the eye clinic. Early detection has major benefits in the outcome of individual patients as has been demonstrated in developed countries.10 However, preventing unnecessary referral to the eye clinic of patients with insignificant eye disease is also important in our setting, as healthcare services are overloaded. Patients were spared a second visit, which translates into cost-saving in transport and hospital visits and less time away from work for employed patients.
Studies conducted both internationally and locally at various levels of care demonstrated that screening people living with diabetes (PLWD) with a fundus camera improved the quality of care. In India, Wadhwani et al. screened PLWD aged 40 years and older using a portable handheld NMFC in screening camps in the slum communities of Delhi between January 2013 and June 201429 of the 9435 patients screened, 1273 (13.5%) had diabetic retinopathy; 351 (3.7%) of these had mild NPDR, 567 (6.0%) had moderate, and 92 (1.0%) had severe NPDR. PDR was noted in 77 cases (0.8%) and 49 (0.5%) had DME. All cases with DR were referred for tertiary care. In Pakistan, Fahadullah et al. also used a NMFC to screen type 2 DM patients for DR between January and May 2015. They screened 2970 eyes of 1485 patients and found DR in 646 (21.8%) of eyes. 30
It has been recommended that effective screening programmes for diabetic eye disease should take place outside of the ophthalmology service. 31 Numerous barriers to effective screening have been identified in various settings, which include access to the programme and resources. 32
A study geographically comparable to ours is one by Mash et al. conducted in the Cape Town metropole. Of 400 individuals who underwent screening, 63% had retinopathy (22% severe nonproliferative retinopathy, 6% proliferative retinopathy) and 15% had maculopathy with 7% of all cases requiring immediate laser treatment. 27 However, only 2.8% of patients in our study required referral for urgent treatment while 17.5% needed routine referral and most of our patients had no retinopathy. The fact that most participants in the study by Mash et al. had Type 2 DM, were middle-aged and hypertensive may partially explain the differences between the two studies. Notwithstanding
the differences, both these local studies have demonstrated that DR screening of patients using a NMFC is an effective way of detecting DR and preventing unnecessary referrals to eye clinics that are already overburdened. Another local study screened 14 541 patients between 2007 and 2010. They calculated a cost-effectiveness ratio of $1206 per blindness case averted which included screening and treatment costs. However, the cost to only screen a patient for DR was a mere $22. 33
A recent study performed in a high-risk diabetic clinic in Soweto, South Africa, concluded that screening with digital nonmydriatic fundus photography was effective in detecting DR in their population, with 28% diagnosed with DR. Of these patients, only 27.02% had Type 1 DM. 34
In the future, it may be possible to further improve the cost-effectiveness of this type of screening by introducing artificial intelligence (AI) into the grading of different types of retinal disease. For instance, a study conducted in China demonstrated that AI-based DR screening had a high sensitivity and specificity in detecting DR. DR was detected in 143 (16.1%) people by ophthalmologists and in 145 (16.3%) participants by AI. 35 In a study from Zambia, retinal images were taken from 1574 PLWD which detected referable DR (moderate NPDR or worse, DME and ungradable images) in 22.5% of eyes and vision-threatening DR in 5.5% of eyes. 28 The area-under-the-curve (AUC) of the AI programme for referable DR was 0.973 (95% CI 0.969 - 0.978) with sensitivity of 92.25% and specificity of 89.04%. The sensitivity for vision-threatening DR was 99.42%.
23 This demonstrates the potential shown by AI technology to reduce preventable blindness in under-resourced countries. A systematic review of seven studies found that a telemedicine programme that involved taking a retinal image and sending it to an ocular imaging facility to assess the severity of DR could potentially result in significant cost savings by improving patients’ working capacity and lowering transport costs. 36 A local study evaluated Google AutoML Vision AI trained using a DR dataset evaluated against realworld images classified by vitreoretinal trained ophthalmologists, resulting in a sensitivity of detecting referable DR of 85.7% (95% CI, 81.0% - 90.5%) and a positive predictive value of 96.3% (95% CI, 92.8%98.1%) making it an attractive option in South Africa. 37
The other major benefit of adopting NMFC screening for DR is the number of
unnecessary referrals to the Ophthalmology service that it prevented. The eye clinic at Tygerberg Hospital has been the busiest outpatient clinic at the facility for many years and is severely overburdened. By preventing the unnecessary referral of 139 patients over a six-month period the use of this type of screening allows for more capacity in the clinic.
This research has certain limitations. Firstly, the retrospective design was not ideal as some important data were not captured. Similarly, the challenge of evaluating the financial impact because of incomplete data. The patient numbers are also quite low, but this was a direct consequence of the Covid-19 pandemic and the periods of lockdown experienced in South Africa. Additionally, other types of diabetes mellitus were not evaluated during this study.
Studies have shown a correlation between nephropathy and retinal disease. 38 Furthermore, it is indicated that there is a high association between HbA1c and retinal disease. 39 Evaluating the correlation between factors like nephropathy, HbA1c and total daily insulin dose was not part of this study. This will be taken into consideration in future research.
The service will be extended to additional cohorts, for example pregnant patients with DM.
Conclusion
NMFC imaging demonstrated value in a low-middle income setting to prioritise cases by detecting significant diabetic retinopathy and avoiding unnecessary referrals to the ophthalmology clinic in patients with no or mild eye disease.
References
1. Saeedi P, Petersohn I, Salpea P, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract 2019;157:107843. doi:10.1016/j.diabres.2019.107843.
2. Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87(1):4-14. doi:10.1016/j. diabres.2009.10.007.
3. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract 2014;103(2):137-149. doi:10.1016/j. diabres.2013.11.002.
4. Gouda HN, Charlson F, Sorsdahl K, et al.
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 22 Original study Non-mydriatic fundus
Burden of non-communicable diseases in subSaharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375-e1387. doi:10.1016/ S2214-109X(19)30374-2.
5. Saloni Dattani, Fiona Spooner HR and MR (2023) “Causes of DP online at OurWorldInData org. R from: “https://ourworldindata. org/ causes of death” [Online R. No Title.
6. Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract 2018;138:271-281. doi:10.1016/j.diabres.2018.02.023.
7. Bigna JJ, Noubiap JJ. The rising burden of noncommunicable diseases in sub-Saharan Africa. Lancet Glob Health. 2019;7(10): e1295-e1296.
8. Klein R, Klein BEK. Diabetic eye disease. The Lancet . 1997;350(9072):197-204.
9. Jampol LM, Glassman AR, Sun J. Evaluation and Care of Patients with Diabetic Retinopathy. N Engl J Med 2020;382(17):1629-1637. doi:10.1056/ nejmra1909637.
10. Chang YC, Wu WC. Dyslipidaemia and diabetic retinopathy. Rev Diabet Stud. 2013;10(2-3):121132. doi:10.1900/RDS.2013.10.121.
11. Li YT, Wang Y, Hu XJ, et al. Association between systolic blood pressure and diabetic retinopathy in both hypertensive and normotensive patients with type 2 diabetes: Risk factors and healthcare implications. Healthc (Switz). 2021;9(5):1-13. doi:10.3390/ healthcare9050580.
12. Yau JWY, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556-564. doi:10.2337/dc11-1909
13. Avogaro A, Fadini GP. Microvascular complications in diabetes: A growing concern for cardiologists Int. J. Cardiol 2019;291:29-35. doi:10.1016/j.ijcard.2019.02.030.
14. Modjtahedi BS, Wu J, Luong TQ, Gandhi NK, Fong DS, Chen W. Severity of Diabetic Retinopathy and the Risk of Future Cerebrovascular Disease, Cardiovascular Disease, and All-Cause Mortality. Ophthalmology. Published online 2021. doi:10.1016/j.ophtha.2020.12.019.
15. Brownrigg JRW, Hughes CO, Burleigh D, et al. Microvascular disease and risk of cardiovascular events among individuals with type 2 diabetes: A populationlevel cohort study Lancet Diabetes Endocrinol 2016;4(7):588-597. doi:10.1016/ S2213-8587(16)30057-2.
16. Xie J, Ikram MK, Cotch MF, et al. Association of diabetic macular edema and proliferative diabetic retinopathy with cardiovascular disease a systematic review and metaanalysis. JAMA Ophthalmol. 2017;135(6):586593. doi:10.1001/jamaophthalmol.2017.0988.
17. Modjtahedi BS, Wu J, Luong TQ, Gandhi
NK, Fong DS, Chen W. Severity of Diabetic Retinopathy and the Risk of Future Cerebrovascular Disease, Cardiovascular Disease, and All-Cause Mortality. Ophthalmology (Rochester, Minn). Published online 2020. doi:10.1016/j.ophtha.2020.12.019.
18. Liu Y, Swearingen R. Diabetic Eye Screening: Knowledge and Perspectives from Providers and Patients. Curr Diab Rep 2017;17(10). doi:10.1007/s11892-017-0911-2.
19. Sheppler CR, Lambert WE, Gardiner SK, Becker TM, Mansberger SL. Predicting Adherence to Diabetic Eye Examinations: Development of the Compliance with Annual Diabetic Eye Exams Survey. Ophthalmology. 2014;121(6):12121219. doi:https://doi.org/10.1016/j. ophtha.2013.12.016.
20. Hipwell AE, Sturt J, Lindenmeyer A, et al. Attitudes, access and anguish: a qualitative interview study of staff and patients’ experiences of diabetic retinopathy screening. BMJ Open. 2014;4(12):e005498. doi:10.1136/ bmjopen-2014-005498.
21. Burgess PI, Msukwa G, Beare NAV. Diabetic retinopathy in sub-Saharan Africa:| c. BMC Medicine. 2013;11(1). doi:10.1186/1741-7015-11-157.
22. Panwar N, Huang P, Lee J, et al. Fundus photography in the 21st century - a review of recent technological advances and their implications for worldwide healthcare. Telemed J E Health. 2016;22(3):198-208. doi:10.1089/tmj.2015.0068.
23. Bellemo V, Lim ZW, Lim G, et al. Artificial Intelligence Using Deep Learning to Screen for Referable and Vision-Threatening Diabetic Retinopathy in Africa. SSRN Journal. Published online 2020. doi:10.2139/ssrn.3324738.
24. Ting DSW, Cheung CYL, Lim G, et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMAJ. Am. Med. Assoc. JAMA. 2017;318(22):2211-2223. doi:10.1001/jama.2017.18152.
25. Lu L, Ren P, Lu Q, et al. Analyzing fundus images to detect diabetic retinopathy (DR) using deep learning system in the Yangtze River delta region of China. Ann. Transl. Med. 2021;9(3):226-226. doi:10.21037/atm-20-3275.
26. DeBuc DC. The Role of Retinal Imaging and Portable Screening Devices in Teleophthalmology Applications for Diabetic Retinopathy Management. Curr. Diab. Rep. 2016;16(12). doi:10.1007/s11892-016-0827-2.
27. Mash B, Powell D, Du Plessis F, Van Vuuren U, Michalowska M, Levitt N. Screening for diabetic retinopathy in primary care with a mobile fundal camera -evaluation of a South African pilot project. S. Afr. Med. J. 2007;97(12):1284-1288. doi:10.7196/SAMJ.203.
28. Solomon SD, Goldberg MF. ETDRS Grading of Diabetic Retinopathy: Still the Gold Standard?
Ophthalmic Research. 2019;62(4):190-195. doi:10.1159/000501372
29. Wadhwani M, Vashist P, Singh SS, et al. Diabetic retinopathy screening programme utilising non-mydriatic fundus imaging in slum populations of New Delhi, India. Trop. Med. Int. Health 2018;23(4):405-414.
30. Fahadullah M, Memon NA, Salim S, et al. Diagnostic accuracy of non-mydriatic fundus camera for screening of diabetic retinopathy: A hospital based observational study in Pakistan. J Pak Med Assoc. 2019;69(3):378-382.
31. Lanzetta P, Sarao V, Scanlon PH, et al. Fundamental principles of an effective diabetic retinopathy screening program. Acta Diabetol. 2020;57(7):785-798. doi:10.1007/ s00592-020-01506-8.
32. Nishantha Piyasena MMP, Murthy GVS, Yip JLY, et al. Systematic review on barriers and enablers for access to diabetic retinopathy screening services in different income settings. PLoS One. 2019;14(4). doi:10.1371/ journal.pone.0198979.
33. Khan T, Bertram MY, Jina R, Mash B, Levitt N, Hofman K. Preventing diabetes blindness: Cost effectiveness of a screening programme using digital non-mydriatic fundus photography for diabetic retinopathy in a primary health care setting in South Africa. Diabetes Res. Clin. Pract. 2013;101(2):170-176. doi:10.1016/j. diabres.2013.05.006.
34. De Jager W MI. Determining the prevalence of diabetic retinopathy in a high-risk diabetic clinic in Soweto, South Africa, using digital colour fundus photographs for screening. SA Ophthalmology Journal. 2023;18(1):10-14.
35. He J, Cao T, Xu F, et al. Artificial intelligencebased screening for diabetic retinopathy at community hospital. Eye (Basingstoke) 2020;34(3):572-576. doi:10.1038/ s41433-019-0562-4.
36. Avidor D, Loewenstein A, Waisbourd M, Nutman A. Cost-effectiveness of diabetic retinopathy screening programs using telemedicine: A systematic review. Cost Effect Resour Alloc. 2020;18(1):1-10. doi:10.1186/ s12962-020-00211-1.
37. Abdoola F KC. Evaluation of Google AutoML Vision artificial intelligence in detecting referable diabetic retinopathy in South Africa. SA Ophthalmology Journal. 2023;18(1):17-21.
38. Antwi-Boasiako C, Obeng KA, Amissah-Arthur KN, et al. Association between albuminuria and retinal microvascular dysfunction in type 2 diabetes with and without hypertension. Diabetes Epidemiology and Management. 2023;11:100139. doi:10.1016/j.deman.2023.100139.
39. Thomas RL, Distiller L, Luzio SD, et al. Incidence and progression of diabetic retinopathy within a private diabetes mellitus clinic in South Africa. J Clin Endocrinol Metab. 2015;20(3):28-34. doi:10.1080/16089677.2015.1090159.
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 23 Original study Non-mydriatic fundus

Frosted branch angiitis: A rare blinding vasculitis
in three children presenting acutely to Red Cross Children’s War Memorial Hospital…unmasking possible mumpsassociated sequelae in the unvaccinated?
N Narainswami, FCOPH (SA), MMED (UKZN), Dip Oph (SA), Dip HIV Man (SA), MBBCH (Wits); Fellow Paediatric Ophthalmology and Strabismus, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0002-5495-7153
N Freeman, MB ChB (Stell), FC Ophth (SA), MMed (Ophth) (Stell); Consultant, Paediatric Ophthalmology and Strabismus, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0002-1110-9795
T Seobi, MBBCH (Wits), MMED(Wits), FCOphth(SA); Consultant, Paediatric Ophthalmology and Strabismus, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa.
ORCID: https://orcid.org/0000-0001-7125-4217
Corresponding author ; N Narainswami, e-mail: neerannarainswami@gmail.com
Abstract
Introduction: Frosted branch angiitis is a rare clinical entity with less than two hundred and fifty cases described in the literature. This report seeks to describe the clinical features and management of this devastating retinal vasculitis presenting in three children under five years of age to Red Cross War Memorial Children’s Hospital (RCWMCH) just weeks apart.
Materials and Methods: Observational case report of three patients.
Case report: Three otherwise healthy children presented within the space of one month (May -June) to our eye clinic with a history of sudden, painless and profound vision loss post a flu-like prodrome a few weeks prior. An extensive infective and inflammatory work up proved negative except for positive mumps serology in one patient with an antecedent history of mumps. None of the children were previously vaccinated against mumps. Despite initial limited and poor response to aggressive systemic steroid therapy, escalation to infliximab infusion therapy seems to be assisting visual recovery in all three patients.
Conclusion: Mumps- associated retinal vasculitis can present as a devastating blinding disease. With the concurrent surge in ‘epidemic parotitis’ in South Africa and the absence of any
Case Report
Three previously healthy boys from the Western Cape ranging from two to four years presented within the space of one month to the eye clinic at Red Cross Children’s Hospital with a history of
other aetiologic factor other than mumps proven on serology it is possible that these cases may represent a spectrum of complications in unvaccinated children especially under the age of five years. The possible diagnosis of mumps- associated retinal vasculitis carries a significant public health concern needing to be highlighted and addressed by our health care sector. This is especially critical as there is currently no mumps vaccine freely available in the state sector in South Africa. Consideration of re-instituting the mumps vaccine may have to be undertaken to mitigate consequences in young children.
Keywords: frosted branch angiitis, retinal vasculitis, vaccine, epidemic parotitis, mumps.
Acknowledgements: Department of Neurology RCWMCH, Department of Virology RCWMCH, Dr J Steffen Department of Ophthalmology Groote Schuur Hospital, Dr W Huwaidi Registrar UCT Ophthalmology, Dr H Kassa Department of Rheumatology RCWMCH.
Conflicts of interest: None.
Ethical considerations: Written informed consent was obtained from the parents for use of clinical data and images.
sudden painless blindness post flu-like symptoms a few weeks prior.
Case 1: A three-year-old unvaccinated child from Atlantis, presented with profound bilateral painless loss of vision of PLP/? NPL OU without navigation.
Case 2: A four-year-old child from Hout Bay with a history suggestive of mumps including parotid swelling that was treated at home conservatively a few weeks prior. Vision was HM OU with limited navigation.
Case 3: A two-year-old from Athlone
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 25 Case series Frosted branch angiitis
presented a week later with bilateral PLP vision with poor navigation.
None of the children lived in the same district and there was no travel history nor any other contributory history to account for a common environmental exposure.
All three children presented with profound bilateral vision loss and limited navigation. Pupil reactions were sluggish in all three children. They were otherwise systemically well. Further examination of the anterior segment was unremarkable in all three. Dilated fundus exam revealed a striking frosted branch angiitis picture with severe vascular sheathing, relatively minimal vitritis and a punctate retinitis in the mid to peripheral retina in the first two cases. The third and youngest child had marked vascular sheathing falling short of being described as frosted branch, but with a similar punctate retinitis extending into the posterior pole. No occlusive disease was clinically evident in all six eyes of three cases. All three had diffuse retinal oedema with associated macula oedema. Optic discs were pink without any disc swelling.
Infective work-up including HIV testing, TB Mantoux, CXR and TB gene expert were all negative as were TORCH screenings. Covid nasal PCR was negative in all three cases. Nasopharyngeal swabs for respiratory viruses (RSV/Coronavirus/adenovirus) were non-contributory. Inflammatory markers (ESR/CRP/ENA panel) were all within normal range and an extensive autoimmune panel (SLE/Bechets/neuro-sarcoid) in each child turned up negative. MRI brain and orbits were within normal limits for all three boys with no evidence of a cerebral vasculitis nor demyelination.
The first two children were commenced on intravenous (IV) acyclovir for at least 24 hours prior to an IV pulse of methylprednisolone of five days duration. This was to empirically cover for any possible herpetic cause of vasculitis while awaiting infective work-up. Serology for syphilis, toxoplasmosis, HSV, VZV, CMV, Rubella, anti-streptolysin O were all negative. Mumps serology, which is not routinely available in state service, was also deemed necessary due to the parallel mumps outbreak in South Africa. Only one swab in Case 1 was positive for rhinovirus, but rhinovirus is not associated with retinal vasculitis. Lumbar puncture and CSF analysis revealed an absence of cells on chemistry and all three children were negative for viral (HSV/CMV/VZV/ mumps) PCR and VDRL testing. Post IV pulsing the frosted branch picture showed dramatic resolution on fundoscopy, but
no concurrent visual gains were seen in the first month. Electrophysiologic testing revealed markedly diminished activity in all three cases. Clinically, there appeared to be narrowing of some of the retinal vessels in all three children with stippling hyperpigmentation in the periphery as vascular sheathing and retinal oedema resolved. We decided to test vitreous fluid and perform fundus fluorescein angiography (FFA) to correlate our clinical findings and assess if there was any viable retina worth salvaging in each child. FFA showed a remarkably well-preserved retinal vasculature with normal calibre vessels and no occlusive vasculitis nor stasis despite the initial striking funduscopic signs. Macular perfusion was good in all three cases and no capillary drop out was noted. The last child to present (Case 3) showed some late leakage from temporal retinal vessels at the watershed zone. A trial of intravitreal triamcinolone acetate was given in one eye of Case 1 with the poorest baseline vision to assess response.
Vitreous tap specimens were negative for viral (HSV/VZV/CMV) PCR including mumps. All three children remained on gradual oral prednisone taper over four weeks. Visual gains however seemed to lag and remained underwhelming. Case 1 remained light perception with poor projection and navigation a month post intravitreal steroid injection. Intraocular pressure in the injected eye increased in comparison to the fellow eye but was controlled adequately on antiglaucoma drops.
Case 2 with antecedent history of mumps also showed a marked improvement in vasculitis activity with no leakage on FFA. At one month his vision was PL with assisted navigation. He was able to confidently pick up large high contrast objects presented to him and but unable to recognise faces of family members.
Vision in the third case also improved to navigating independently though he remained unable to identify high contrast optotypes at one month.
At this point mumps serology was positive (both IgG and IgM) in one child (Case 2).
At two months post presentation there was no further appreciable visual improvement, despite improvement in inflammatory activity. Further multidisciplinary consensus determined that an escalation to a trial of IV immunemodulatory therapy (infliximab) was a reasonable treatment option. The attendant complications of cataract and glaucoma made continued steroid therapy
unfeasible beyond two months. The delayed presentation coupled with the improved ocular inflammatory activity on steroid therapy made the diagnosis of a post-infectious immune hypersensitivity phenomenon most likely.
One month after the first infliximab infusion there was an improvement in vision noted with all three cases able to navigate independently and Case 3 able to identify and pick up large silver balls. The decision was made to complete three doses of IV infliximab and commence oral 5-7.5mg weekly methotrexate after the first infusion as well as to continue low dose oral 5mg oral prednisolone maintenance. Visual gains in all three cases were noted after each infliximab infusion with vision in all three cases improving dramatically to at least 6/24 (identify and pick up 100s and 1000s) after the third infusion.
Discussion
Mumps is a viral infection caused by a paromyxovirus, a member of the Rubulavirus family.1 Commonly affecting children under ten years it usually runs a mild and self-limiting course.1 Older children and adults can also be uncommonly affected. Mumps infection in childhood generally confers lifelong immunity. Ocular manifestations of mumps are rare but well documented with acute dacryoadenitis being the most common finding followed by optic neuritis, though this is usually in the setting of a meningo-encephalitis. 2,3
Frosted branch angiitis (FBA) is a rare clinical entity with less than 60 known cases in the world literature and only a few attributable to mumps.4 Primary (idiopathic) FBA presents as a florid translucent retinal perivascular sheathing of both arterioles and venules (though typically a predominant periphlebitis and variable vitritis. It has a variable course, typically affecting young children as low immunoglobulin levels at this age is postulated to be inadequate to suppress an immune response to any number of infectious agents. Secondary FBA occurs in the setting of ocular and systemic viral or auto-immune disease. CMV retinitis in the setting of HIV is a classic example with CMV known to have a tropism for endothelial cells. The deposition of antigen-antibody complexes, as is also seen in auto-immune disease such as SLE, is thought to incite a vasculitis. The frosted branch picture that is sometimes seen in leukaemia and lymphoma is due to infiltration of actual malignant cells.
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 26 Case series
branch angiitis
Frosted

Following the reports of clusters of mumps outbreaks earlier in February this year, the National Institute of Communicable Diseases (NICD) of South Africa confirmed an outbreak on 11 May 2023 with the annual percent positivity for mumps IgM reaching 69% (prior having peaked at 39% in 2019).1 Annual percentpositivity for mumps IgM tests by age category shows marked increases in percent-positivity in the one-four-year age category (84% in 2023) and the five-nineyear age category (83% in 2023), followed by the 30-34-year age category (67%) and 10-14-year age category (54%) (Figure 1).1
All three children presented during the same period that South Africa recorded its highest ever percent positivity for mumps IgM testing in the under fiveyear age category. Case 2 had parotid swelling in keeping with clinical signs

of mumps. While immunisations were up to date for all three children, none received the mumps vaccine as it is no longer part of the extended programme for immunisation (EPI) in the state sector and is only available in the private health sector in South Africa.1 None of the parents had been aware that the EPI no longer offered mumps vaccine coverage.
There is no known standard of care for FBA. Systemic corticosteroid therapy has been employed in most cases of primary FBA with documented improvements in anatomic and functional outcomes.6-8
With exceptionally few isolated case studies on mumps-associated vasculitis in the literature, 6,7 a multidisciplinary approach was deemed necessary to guide therapeutic interventions. The negative viral (mumps, VZV, HSV, CMV, rubella) PCR results on vitreous tap sampling in all

three children coupled with positive IgG mumps serology in one child, seem to support an underlying immune-mediated response to an inciting antigen in the eye rather than the presence of ocular infection. This finding has been suggested in earlier case reports. 2,8 The delayed onset of vision loss following the flu-like symptoms in two of the three cases also supports the concept that the immune mediated response may cause the vison loss and not the virus itself.
Only one child (Case 2) had positive IgG and IgM serology for mumps. All three children were immune competent. Serological methods and test kits for mumps vary considerably in their sensitivity and specificity with some immunofluorescent assays detecting as few as 12%-15% of confirmed mumps cases.9 A negative mumps PCR in blood,

Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 27 Case series Frosted branch angiitis
Figure II: FFA showing vasculitis and leakage of temporal retinal vessels left eye of Case 2 and relatively well-preserved macula perfusion both eyes.
Figure I: Annual percent-positivity for mumps IgM tests by age category (in years) illustrated from national public sector laboratory data from the NICD surveillance data warehouse for the epidemiological week 01 in 2013 (ending 05/01/2013) to week 12 2023 (ending 31/03/2023).



nasopharyngeal swab and vitreous fluid does not exclude the diagnosis of mumps as the virus does not linger beyond few weeks post infection.9 This is important when considering FBA as an immunemediated sequelae of mumps infection as it does not require the detection of mumps via PCR testing to consider the diagnosis.
Bilateral neuro-retinitis post mumps with subsequent vision loss has been reported in at least six cases with improved visual acuities to normal or near normal in most cases.6-8 The pattern in our three patients is however different with there being an overwhelming retinal vasculitis with punctate retinitis and no features of optic nerve involvement clinically. The MRI imaging also showed no ON involvement. Also contrary to the few anecdotal cases in the literature initial visual recovery in our patients was underwhelming from PL vision to PL vision with some projection after intravenous steroid pulsing.6,10 Sayadi et al reported the use of hyperbaric oxygen therapy in managing mumps-associated retinal vasculitis in a child with notable improvements in visual activity. 3 This modality of treatment is unfortunately not readily available to the public health sector in South Africa.
One eye of one child (Case1) with PLP/ NPL vision was injected with intravitreal triamcinolone but showed no gain in vision post injection. Intraocular pressure did however elevate but was well controlled on topical agents. With minimal visual recovery after two months of oral taper of prednisone, the decision was made to escalate to a trial of infliximab infusion therapy to assess response in visual recovery. This was new unchartered territory in terms of treatment being guided mostly by a few anecdotal case studies and no large evidence -based trials. Infliximab, a chimeric mouse/ human monoclonal anti-TNF antibody, was chosen based on its suggested effect for treatment of refractory childhood uveitis
including juvenile idiopathic arthritis and its relatively low rate of treatmentending adverse events.11,12 Notable visual gains both subjectively and objectively were evident in all three cases with each successive infliximab infusion.
Conclusion
Primary frosted branch angiitis can present as a fulminating blinding vasculitis.14 South Africa was in the grip of a mumps outbreak as confirmed by NICD in May 2023. All three children were unvaccinated against mumps. Positive mumps serology in the absence of any other systemic or ocular cause seems to suggest that these cases represent an immune-mediated response to the mumps virus in the unvaccinated.
While a novel or as yet unknown infectious agent cannot definitively be ruled out, this seems unlikely in the face of a parallel mumps outbreak.
Careful consideration by all stakeholders may need to be taken into possibly re-introducing the mumps vaccine as part of the immunisation schedule in South Africa.
References
1. https://www.nicd.ac.za/confirmation-ofmumps-outbreak-south-africa-11-may-2023/.
2. Walker S et al. Frosted branch angiitis: a review Eye 18,527533@0 04 eye, 18257-5333.
3. Sayadi J, Ksiaa I, Malek I, Ben Sassi R, Essaddam L, Khairallah M, Nacef L. Hyperbaric Oxygen Therapy for MumpsAssociated Outer Retinitis with Frosted Branch Angiitis. Ocul Immunol Inflamm 2022 May 19;30(4):1001-1004. doi: 10.1080/09273948.2020.1841243. Epub 2021 Feb 5. PMID: 33545017.
4. Hedayatfar A, Soheilian M. Adalimumab for treatment of idiopathic frosted branch angiitis: a case report. J Ophthalmic Vis Res 2013; 8(4): 372-375.
5. Neri P, Aljneibi S, Pichi F. Rescue Treatment with Infliximab for a Bilateral, Severe, Sight Threatening Frosted Branch Angiitis
Associated with Concomitant Acute Onset of Presumed Dermatomyositis. Ocul Immunol Inflamm. 2022; 8: 1-5. doi:10.1080/09273948.2 022.2057333
6. Khubchandani R, Rane T, Agarwal P, Nabi F, Patel P, Shetty AK. Bilateral Neuroretinitis Associated with Mumps. Arch Neurol 2002;59(10):1633–1636. doi:10.1001/ archneur.59.10.1633.
7. Ali A, Ku JH, Suhler EB, Choi D, Rosenbaum JT. The course of retinal vasculitis. The British journal of ophthalmology. Jun 2014;98(6):785-789 George RK, Walton RC, Whitcup SM,2Nussenblatt RB. Primary retinal vasculitis. Systemic associations and diagnostic evaluation. Ophthalmology. Mar 1996;103(3):384-389.
8. Rosenbaum JT, Ku J, Ali A, Choi D, Suhler EB. Patients with retinal vasculitis rarely suffer from systemic vasculitis. Semin Arthritis Rheum. Jun 2012;41(6):859-865.
9. Rota JS, Rosen JB, Doll MK, McNall RJ, McGrew M, Williams N, Lopareva EN, Barskey AE, Punsalang Jr A, Rota PA, Oleszko WR. Comparison of the sensitivity of laboratory diagnostic methods from a wellcharacterized outbreak of mumps in New York City in 2009. Clin Vaccine Immunol . 2013 Mar;20(3):391-6.
10. Dorairaja T, Yuen GS, Rahmat J. Idiopathic Frosted Branch Angiitis In Paediatric Patients: Case Series . Ijmms. 2019 May;427-32.
11. Walton RC, Ashmore ED. Retinal vasculitis. Curr. Opin. Ophthalmol Dec 2003;14(6):413-419.
12. Abu El-Asrar AM, Herbort CP, Tabbara KF. Retinal vasculitis. Ocul Immunol Inflamm. Dec 2005;13(6):415-43.
13. Levy-Clarke GA, Nussenblatt R. Retinal vasculitis. Int. Ophthalmol. Clin. Spring 2005;45(2):99-11.
14. Rodriguez A, Calonge M, Pedroza-Seres M, et al. Referral patterns of uveitis in a tertiary eye care center. Arch. Ophthalmol. May 1996;114(5):59.
15. Hughes EH, Dick AD. The pathology and pathogenesis of retinal vasculitis. Neuropathol Appl Neurobiol Aug 2003;29(4):325-340.
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 28 Case series Frosted branch angiitis
Figure III: Dramatic reduction in swelling, and frosted branch picture post IV steroid pulse.





When inflammation hits, strike back with Loteprednol Etabonate

References:




Site-specific, high-anti-inflammatory e cacy 1-3
Improved safety profile 1, 2, 4





Modulates and inhibits early-and late-phase inflammatory mediators for multi-symptom relief 1, 5
Lower risk for IOP elevation in short and long-term use 5





Engineered to adhere to the ocular surface, for post-operative inflammation 6-8
Uniform dose delivery, in every drop 7, 9
1. Pavesio CE, et al. Treatment of ocular inflammatory conditions with loteprednol etabonate. Br J Ophthalmol 2008;92:455– 459. 2. Dell SJ, et al. A randomized, double-masked, placebo-controlled parallel study of 0.2% loteprednol etabonate in patients with seasonal allergic conjunctivitis. J Allergy Clin Immunol 1998;102:251-5. 3. Gong L, et al. Loteprednol Etabonate Suspension 0.2% Administered QID Compared With Olopatadine Solution 0.1% Administered BID in the Treatment of Seasonal Allergic Conjunctivitis: A Multicenter, Randomized, Investigator Masked, Parallel Group Study in Chinese Patients. Clin Ther. 2012;34:1259–1272. 4. Comstock TL, et al. Advances in Corticosteroid Therapy for Ocular Inflammation:Loteprednol Etabonate. Int J Inflam 2012; 789623:1-11. 5. Ilyas H, et al. Long-term safety of loteprednol etabonate 0.2% in the treatment of seasonal and perennial allergic conjunctivitis. Eye Contact Lens 2004;30(1):10-13. 6. Lotemax® Ophthalmic Gel package insert, August 2022. 7. Fong R., et al. Loteprednol etabonate gel 0.5% for postoperative pain and inflammation after cataract surgery: results of a multicentre trial. Clin Ophthalmol. 2012;6:1113-1124. 8. Shaikh R et al. Mucoadhesive drug delivery systems. J Pharm Bioallied Sci. 2011;3:89-100. 9. Co ey MJ and Davio SR. Presented at: ARVO 2012; May 2012; Ft. Lauderdale, FL. Poster D1143.
S4 Proprietary name and dosage form: Lotemax ophthalmic suspension, eye drops. Composition: Each 1 ml contains: Loteprednol Etabonate 5,00 mg (0,5 % m/v) and Benzalkonium chloride (preservative) 0,01 % m/v Pharmacological classification: A 15.2 Ophthalmic preparations with corticosteroids. Registration number: 37/15.2/0588. S4 Proprietary name and dosage form: Alrex Ophthalmic Suspension. Composition: Each 1 ml contains: Loteprednol etabonate 2,00 mg (0,2 % m/v) and Benzalkonium chloride (preservative) 0,01 % m/v Pharmacological classification: A 15.2 Ophthalmic preparations with corticosteroids. Registration number: 38/15.2/0203. S4 Proprietary name and dosage form: Lotemax Ophthalmic Gel. Composition: Loteprednol etabonate 5,00 mg (0,5 % m/v) and Benzalkonium chloride (preservative) 0,003 % m/v Pharmacological classification: A 15.2 Ophthalmic preparations with corticosteroids. Registration number: 51/15.2/9036. For full prescribing information, refer to the professional information as approved by the South African Health Products Regulatory Authority (SAHPRA) © 2024 Bausch & Lomb Incorporated or its a liates. ® / TM denote trademarks of Bausch & Lomb Incorporated or its a liates. Soflens (Pty) Ltd. Reg. no.: 1968/011787/07. 254 Hall Street, Centurion, 0157.
+27 10 025
co.za.
0.5
0.2
Tel:
2100. www.bausch.
BL619/23 NEW GEL 0.5 %
%
%
Penetrating orbital injury causing a prepontine haemorrhage and abducens palsy
S Rashid, MD (KCMUCo); Division of Neurosurgery, University of Cape Town, Cape Town, South Africa.
https://orcid.org/0000-0002-7871-3399
B Kgaodi, MBBS (UB), FC Neurosurg (SA), Mmed Neurosurgery (UCT); Department of Ophthalmology, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa
https://orcid.org/0009-0009-0858-2336
C Tinley, MBChB, FRCOphth (London); Division of Ophthalmology, Groote Schuur Hospital, University of Cape Town , South Africa.
https://orcid.org/0000-0001-5817-7122
N Enslin, BPhyT, MBChB (UP), MMed Neurosurgery (UCT), FC Neurosurg (SA); Division of Neurosurgery, University of Cape Town, Neurosciences Institute, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa
https://orcid.org/0000-0002-6384-7781
A Figaji, MBChB, MMed Neurosurgery (UCT), FC Neurosurg (SA), PhD, BPhyT, MBChB (UP), MMed Neurosurgery (UCT), FC Neurosurg (SA); Division of Neurosurgery, University of Cape Town, Neurosciences Institute, Red Cross War Memorial Children’s Hospital, Cape Town, South Africa
https://orcid.org/0000-0002-3357-6490
Corresponding author: Sakina M Rashid, email: sakina.rashid@ymail.com
Abstract
Background: Penetrating traumatic brain injury (TBI) in children is rare, but potentially fatal and is easily missed. We present an illustrative case of an 11-year-old female who sustained a potentially fatal penetrating orbital injury, which was missed at her index presentation. A sharp object was unintentionally thrust into her left eye, and she presented two days later with an abducens palsy that prompted a computed tomography (CT) scan of her brain. A small left pre-pontine hyperdensity was overlooked. Review at her routine follow-up prompted further magnetic resonance imaging (MRI).
Results: MRI slices through the midbrain and posterior fossa clearly delineated the transorbital intracranial tract with brainstem penetration. Three-dimensional reconstruction of the images demonstrated trajectory through the left superior orbital fissure.
Introduction
Trauma remains the leading cause of morbidity and mortality worldwide in older children and young adults. South Africa has a significant burden of trauma, with approximately 30 000 trauma related deaths annually.1
Traumatic brain injuries (TBIs) are a common cause of poor outcomes: at one high volume provincial trauma centre, TBIs accounted for 32.7% of all trauma mortality; penetrating injuries had a 20% mortality overall. 2
Penetrating brainstem injuries are often fatal. 3 Survival following transorbital pontine injuries has been reported in literature; however, this is rare 3 because of
Conclusion: While penetrating orbital injuries may be obvious on initial presentation, the external signs of significant intracranial penetration can be subtle in children. Awareness of the risk is key to appropriate investigation. A thorough review of the presenting history, detailed neurological examination, and consideration of the involved anatomy reduces the chances of missed injury. With respect to transorbital injury, the conical shape of the orbit tends to direct penetrating objects towards high-risk anatomical regions.
Keywords: penetrating head injury, orbital trauma, abducens palsy, traumatic brain injury, children.
Funding: Not applicable.
Declarations: The authors declare no conflict of interest.
the complex neural and vascular anatomy in a compact area.4
While penetrating TBIs are often obvious on presentation, because of the nature of the injury and the presence of open wounds and foreign material, patients may also present with occult injuries. This is most relevant to childhood injuries because the thinner scalp and bone protecting the central nervous system are more easily breached. Therefore, a high index of suspicion is needed to identify penetrating intracranial injury where the external manifestation is subtle.
This case illustrates the potential for high-risk intracranial injury with minimal external signs.
Statement of ethics
Informed consent and assent were obtained from the mother and the child respectively to proceed with writing and publishing this report. Every effort has been made to exclude information that may identify the child.
Case presentation History
An 11-year female was looking through a keyhole of a door, when her friend on the other side of the door forcefully directed a sharp thin object through the keyhole and unintentionally into her left orbit. She experienced immediate, severe pain in her left eye that settled over some time. On presentation two days later to our
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 30 Case report Pre-pontine haemorrhage and abducens palsy
hospital, she complained of headaches, diplopia, a weak left leg and three episodes of vomiting since the incident.
Examination
On examination, her Glasgow Coma Score (GCS) was 15/15. There was mild left periorbital swelling and a small subconjunctival haemorrhage. Examination of the extraocular movements showed a left sixth nerve palsy. There was no motor weakness noted on lower limb examination; however, proprioception was decreased in the left lower limb. The rest of her examination was unremarkable.
Initial imaging: Computed Tomography (CT) scan
During her index presentation, she underwent a CT scan of the head to assess the extent of her injury and to rule out any retained foreign material in the orbit. There was a hyperdensity anterior to the pons of the brainstem on the left which was initially overlooked (Figure 1). The impression was a sixth nerve injury resulting in a medially deviated left eye. No foreign material was noted in the orbit.

Clinical course
Following her initial presentation, she was admitted for close observation to ensure there was no progression of her symptoms. After 48 hours she was discharged for regular out-patient follow-up. As there was no retained foreign material or any open wounds, no further interventions were instituted during the initial admission. A penetrating brain stem injury had not been considered initially, largely due to how well the child was, and the physical examination signs were thought to be explained by an isolated sixth cranial nerve injury.
During her first clinic follow-up, she was reviewed, and the concern was raised

that the subtle hyperdensity on the head CT scan represented subarachnoid blood or contusion, which implied that there was intracranial injury that needed further investigation. Re-examination of the extraocular movements showed a persistent left sixth nerve palsy (Figure 2). A magnetic resonance imaging (MRI) scan was performed to delineate the extent of injury.
Magnetic Resonance Imaging (MRI) findings
MRI of the brain three weeks post-injury showed a tract with old blood products that traversed the left lateral pons and the middle cerebellar peduncle, extending into

the white matter tracts of the cerebellum (Figure 3). Also noted upon closer examination of the extraconal fat in the left eye was a region of hypodensity between the medial and inferior rectus muscles, which was the likely path of the sharp penetrating object. No false aneurysm was observed.
Clinical course – treatment and outcome
She was followed-up regularly in our outpatient service. The sixth nerve palsy completely resolved over the next few months.
Discussion
Penetrating head injuries are often obvious on initial presentation and rare in children. However, when they do occur, they can present as fatal incidences or with extremely subtle findings that are overlooked by the treating doctor. A combination of physical examination findings, potential trajectory of the penetrating object and radiographic findings can guide the diagnosis and further management of such injuries.
One such entry into the cranium is the orbital route. Sun et al reviewed the variety of everyday objects that have resulted in penetrating pontine injuries through the orbit. Survival is often accompanied by significant neurological fall out. The orbit is a high-risk area because it is a soft tissue corridor into the cranial vault, where its conical shape directs penetrating objects towards its apex and therefore, towards the brainstem.
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 31 Case report Pre-pontine haemorrhage and abducens palsy
Figure 1: CTB demonstrating pre-pontine hyperdensity.
Figure 2: A – Primary position, B – Right gaze, C – Left gaze.
Figure 3: Susceptibility Weighted Imaging (SWI) of the posterior fossa demonstrating the tract through the brain stem and cerebellum.
The sixth nerve has the longest path of any of the cranial nerves. Within the orbit, the sixth nerve innervates the lateral rectus muscle which abducts the eye and allows lateral gaze of the ipsilateral eye. MRI of the orbit in this case showed a hypodensity in the fat between the medial and inferior rectus muscle, away from the more lateral path of the sixth nerve within the orbit. If the sixth nerve was injured in the orbit, it is likely the injury was more proximal, as the nerve traversed the superior orbital fissure.
The second site of the sixth nerve that may have been injured by the projectile is within the cavernous sinus. It is the only cranial nerve that runs through the cavernous sinus that is not within the fold of dura forming its lateral wall. That could explain why an isolated sixth nerve palsy was noted with sparing of the third, fourth and fifth (VI and VII) nerves. The internal carotid artery and its sympathetic plexus lie in close proximity to the sixth nerve in the cavernous sinus. The patient’s examination excluded a Horner’s syndrome or any neurological fall-out attributable to a carotid artery injury therefore further imaging to assess this was not undertaken. It should be noted however that a luminal injury of the carotid artery would place the patient at risk for pseudoaneurysms later in life. 5,6
As the sixth nerve runs up the clivus and over the petrous apex, it turns 90 degrees to enter Dorello’s canal. The projectile may have scraped across the petroclinoid ligament and the sixth nerve prior to continuing its postero-inferior course into the brainstem at the level of the pons.
The MRI of the midbrain and posterior fossa demonstrate the path that the trajectory took, improved by the threedimensional reconstruction of the images. It appears the likely point of the sixth nerve’s injury was at the left superior orbital fissure (Figure 4). The reconstructed images also place into context how fortunate the child was not to have sustained injury to several critical neural and vascular structures in close proximity to the tract, including the basilar artery and its branches perfusing the brainstem (Figure 5).
The sixth nerve nucleus is spared, and the path is generally away from the sixth nerve fibres within the brainstem, being superior and lateral to the nucleus. However, it is not impossible to exclude local oedema or irritation due to subarachnoid blood. The patient’s gradual recovery of the sixth nerve function indicates that it was likely a neuropraxia versus an axonotmesis or neurotmesis.

Fortunately, there was no vascular injury of the basilar artery or its branches, which could have led to a devastating outcome through haemorrhage immediately or delayed due to a false aneurysm.
Conclusion
Penetrating brain injury in children is easy to miss. Orbital entry is one such route where significant penetration into critical structures can occur with little external manifestation. A high index of suspicion, along with careful clinical and radiological evaluation, are key to not missing these injuries.
References
1. MRC/UNISA Crime, Violence and Injury Lead Programme. A profile of fatal injuries in South Africa 2008 - Annual Report for South Africa Based on National Injury Mortality Surveillance System (www.ac.za/crime/nimss07.PDF
2. Moodley N B, Aldous C, Clarke DL (2014)
An audit of trauma-related mortality in a provincial capital in South Africa. S. Afr. J. Surg 2014:52(4):101-104 DOI:10.7196/SAJS.1995.
4. MacLean MA, Mukhida K, Shankar JJ, Schmidt MH, Clarke DB (2019) Complete recovery following transorbital penetrating head injury traversing the brainstem: case report. J Neurosurg Pediatr 6;24(6):697-701. https://doi. org/10.3171/2019.6.PEDS19106.
4. Sun G, Yagmurlu K, Belykh E, Lei T, Preul MC (2016) Management strategy of a transorbital penetrating pontine injury by a wooden chopstick. World Neurosurg 1;95:622-e7. https:// doi.org/10.1016/j.wneu.2014.11.002
5. Alfawaz A, Li X, Kénel-Pierre S, Yang J, Rey J, Robinson H. Delayed presentation of a carotid pseudoaneurysm following penetrating neck trauma SAGE Open Med Case Rep 2016 May.
6. Garg K, Rockman CB, Lee V, Maldonado TS, Jacobowitz GR, Adelman MA, Mussa FF. Presentation and management of carotid artery aneurysms and pseudoaneurysms. J. Vasc. Surg. 2012 Jun 1;55(6):1618-22.

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 32 Case report Pre-pontine haemorrhage and abducens palsy
Figure 5: Image reconstruction demonstrating critical neural and vascular structures in close proximity to the tract.
Figure 4: Bony reconstruction demonstrating the trajectory of the projectile.

34 MESSAGE FROM THE PRESIDENT Fostering unity – essential for effective patient care Dr Steven Lapere 36 OSSA CONGRESS NEWS OSSA 2024 Awards 44 CONGRESS CALENDAR 46 PRODUCT NEWS Latanoprostene Bunod – An innovative solution for lowering IOP

FOSTERING UNITY
essential for effective patient care
The beginning of a new year brings hope, opportunity and a chance to look back at the previous year, its highs and lows, and plan accordingly.
We find ourselves in a rapidly changing environment, from technological advancements to patient, government and funder interactions. Now more than ever we require a sense of community and co-operation across all spheres to benefit our patients.
We have a diverse and vibrant ophthalmic community and a dedicated executive committee. These members tirelessly give up their time and expertise to assist in projects to help improve eye care in South Africa.
The OSSA Congress in Gqeberha was a resounding success, with 852 delegates attending. A special thanks to Schalk du Toit and the local organising committee
for hosting an outstanding congress, and to Ryno Kriek and his team for all the logistical arrangements.
The African Ophthalmology Council will be holding its first face-to-face conference in Kigali, Rwanda from 27 to 29 July, and Bayanda Mbambisa, our past president, will be representing OSSA. Anesu Madikane, a member of OSSA exco, is also president of the African Ophthalmology Council Young Ophthalmologists and will be representing OSSA and its Young Ophthalmologists.
The next OSSA congress will be hosted at Sandton Convention Centre from 13 to 15 February 2025, and Barry Payne and the local organising committee are hard at work arranging a diverse line up of international and local speakers. We look forward to seeing you there!
I wish you all the best for this year. If OSSA

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 34 News Message from the President
us.
Town, FC Ophth (SA) (MB ChB Free State President Ophthalmology Society, South Africa steven.lapere@gmail.com
can be of any assistance to matters relating to patient and clinician advocacy, please do not hesitate to contact
Dr Steven Lapere MMed (Ophth) Cape
Photo credit: Shutterstock.com

OSSA 2024 AWARDS
Honouring excellence and innovation


Dr Steven
Lapere
The Ophthalmological Society of South Africa (OSSA) acts as the central body representing most practicing ophthalmologists nationwide.
Prof Graham Barrett, Consultant Ophthalmologist at Lions Eye Institute at Sir Charles Gairdner Hospital in Perth, WA, is a Clinical Professor at the University Department of Ophthalmology was the invited international guest speaker at the 52nd OSSA Congress.
takes helm as President of Ophthalmology Society of South Africa
Dr Steven Lapere has been elected as the President of the Ophthalmology Society of South Africa for 2024. Based in Claremont, Cape Town, he specialises in disorders of the retina. Dr Lapere completed a comprehensive two-year fellowship in Canada focusing on both surgical and medical retina. He conducts surgeries at Peninsula Eye Hospital and the University of Cape Town’s Private Academic Hospital, while also serving as a sessional consultant at Groote Schuur Hospital.

Specialising in cataract and implant surgery, as well as corneal and keratorefractive surgery, he’s a leader in small incision cataract surgery and phacoemulsification.
During the congress, which took place at the Boardwalk Convention Centre in Gqeberha at the close of February 2024, five esteemed
Honouring the legacy of Dr DJ Wood: A Pioneer in South African Ophthalmology
Born in Scotland in 1865, Dr David James Wood earned his medical degree from Edinburgh at 23 and trained in ophthalmology at Moorfields Eye Hospital in London. Due to health reasons, he relocated to Cape Town in 1893, where he practiced until his passing in 1937.
Dr Wood pioneered ophthalmology in South Africa, becoming its first practitioner and securing the first specialist appointment at New Somerset Hospital in Cape Town in 1923. He was appointed the inaugural lecturer in ophthalmology at the University of Cape Town in 1921 and later served as the first president of OSSA in 1931. Throughout his career, he held significant roles in medical associations and councils. Dr Wood’s noteworthy contributions include 17 publications in the British Journal of Ophthalmology, earning him widespread respect. The Ophthalmology Society of South Africa honours his legacy as the father of ophthalmology in South Africa by bestowing the DJ Wood award annually to a distinguished colleague.

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 36 Congress news



professionals in the field of Ophthalmology were recognised for their outstanding commitment to the profession.
AWARDS
DJ Wood Award
The DJ Wood Award stands as the most prestigious accolade that OSSA can confer upon an ophthalmologist.
The qualifying criteria are: Service to OSSA, and/or academic excellence in ophthalmology, which is measured by contributions to the OSSA congresses and other meetings attended by South African ophthalmologists, and/ or consistent and dedicated service to the community.
Winner of the 2024 DJ Wood Award
Prof Linda Visser
Prof Linda Visser graduated from the University of Pretoria in 1987 with an MBChB degree. Inspired by Prof Colin Cook, she pursued ophthalmology after observing his surgeries during her internship at Edendale Hospital in Pietermaritzburg.
She held positions as a medical officer at Edendale from 1989, followed by a registrar role in Durban, culminating in her obtaining FCOphth (SA) and MMed (Ophth) Natal qualifications in 1995. For 25 years, she dedicated herself to the University of KwaZulu-Natal, specialising in vitreoretinal surgery and assuming the role of academic head of Ophthalmology from

2006. She played a pivotal role in establishing the McCord Provincial Eye Hospital in Durban in 2014. In April 2021, she assumed the position of head of Ophthalmology at Stellenbosch University. Prof Visser has held numerous leadership positions and made significant contributions to ophthalmology organisations. She has authored numerous journal articles, supervised dissertations, and is dedicated to mentoring young ophthalmologists.
President’s Award winner
Dr Clive Novis
This year’s recipient, Dr Clive Novis, was honoured for his dedication and

service during his eleven years as a valued member of the South African Ophthalmology Journal team. Dr Novis’s contributions have been invaluable, with his unique writing style and humorous approach being well-received by readers, enriching the publication’s content while maintaining its informative quality.
Dr Novis’s expertise was first witnessed during an Alcon wetlab session for registrars, where he shared insights on the Little Technique for a runaway capsulorhexis. His remarkable commitment to both the publication and the ophthalmology community at large is highly commendable, and his contributions are deeply appreciated.
HUMANITARIAN AWARD
The purpose of the Humanitarian Award is to recognise participation in charitable activities, indigent care, community service and other humanitarian activities. Individuals must perform this service in their capacity as an ophthalmologist.
The following qualifying criteria apply:
The nominee must have shown consistent humanitarian service in Africa for several years, including caring for the indigent, serving disadvantaged communities in urban or rural areas, tending to underprivileged individuals of specific racial or ethnic backgrounds, and providing care in high-risk environments for ophthalmologists.
Service must have been above and beyond the usual service commitment required of an ophthalmologist.

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 38 Congress news

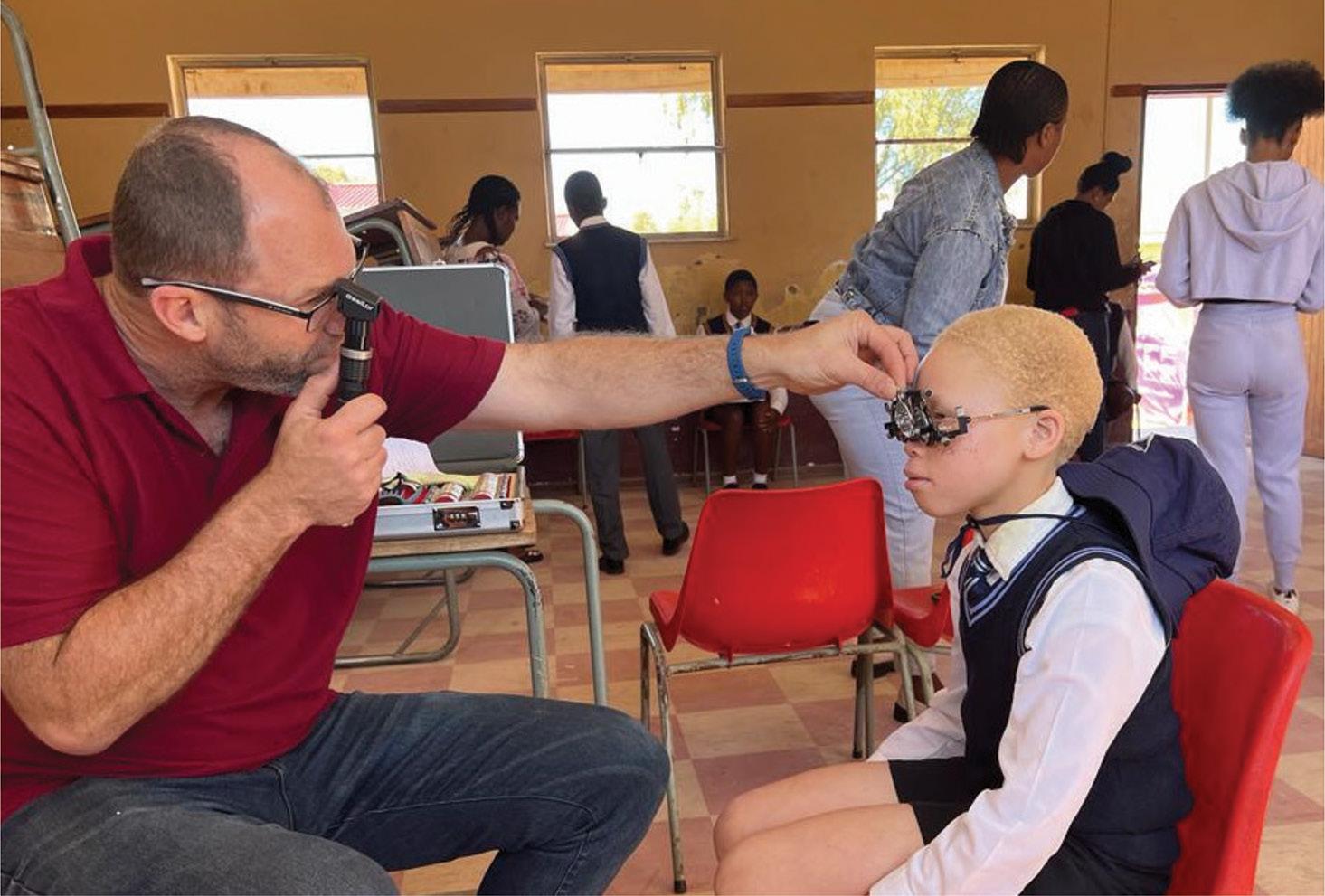


outcomes you can trust


Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 39 Congress news TRUSTED TRIED AND A E L Y E EYLEA ) o e n injection)
Multi-target trap design that inhibits the key drivers of vascular disease, VEGF and PlGF2-4 For full prescribing information, please refer to the Professional Information S4 EYLEA® Solution for Injection. One millilitre solution for injection contains 40 mg aflibercept. Each single dose vial contains an extractable volume of 100 microlitres, equivalent to 4.0 mg aflibercept. Reg. No.: A 46/15.4/0841. Bayer (Pty)Ltd. Reg. No.: 1968/011192/07.27 Wrench Road, Isando, 2609. Tel. +27 11 921 5000. Https://www.eylea.co.za References: 1. Bayer data on file YTD January 2023 2. Papadopoulos N, et al. Angiogenesis 2012;15:171–185. 3. Tadayoni R, et al. Ophthalmologica 2021;244:93–101. 4. Autiero M, et al. J Thromb Haemost 2003;1:1356–1370. PP-EYL-ZA-0159-1
Real-world
>65 million doses worldwide1 >9.4 million years of patient exposure1
Outreach in partnership with the Bona uBuntu Foundation.



The service entails personal sacrifice on the part of the ophthalmologist.
Only one such award may be given to an individual during his or her lifetime.
Humanitarian Award winner
Dr Stephen Cook
Based in East London, South Africa, Dr Stephen Cook is a distinguished figure in ophthalmology, boasting a multifaceted career. As a prominent general ophthalmologist in private practice, he exemplifies healthcare excellence through active involvement in a group practice which has a rich history spanning over 65 years. Dr Cook’s impact reaches beyond his private practice. He lectures at Walter Sisulu University, sharing his expertise with future medical professionals. As a part-time consultant at the Frere-Cecelia Makiwane Hospital Complex, he enhances patient care. Alongside Dr Andrew Boliter, he co-founded the African Eye Foundation, an NPO supporting vital training and pilot
projects that lack commercial sustainability. As the managing director of the East London Day Hospital, he played a pivotal role in its development. Dr Cook’s areas of specialisation encompass glaucoma surgery, vitreoretinal surgery, and oculoplastic surgery, highlighting his diverse skill set within the field of Ophthalmology.
In the field of research, he has been leading the advancement of disease screening methodologies and has championed the development of systems for delivering screening services, often harnessing cuttingedge technology. A notable achievement is the creation of the Glaucoma Score model, a tool designed to aid non-medical graders in identifying glaucoma and informing appropriate referrals to Ophthalmology.
Dr Cook’s dedication to preventive medicine is evident in his ‘Screen for life’ programme, which emphasises the importance of diabetic retinopathy (DR) screening as a biomarker for systemic neurovasculopathy. This programme advocates
for early detection, not only of DR, but also for glaucoma and AMD, underscoring their significance as medical imperatives at the primary care level.
In the surgical arena, Dr Cook specialises in the interface between vitreoretinal and glaucoma surgery, with expertise in the placement of tube shunts during vitreoretinal procedures. His advocacy for the concept of prophylactic tube shunts in non-glaucomatous eyes sparks valuable discussions within the field.
CITIZEN’S AWARD
The following qualifying criterion applies: The nominee is a non-ophthalmologist who has demonstrated a pattern of dedicated service to eye patients during the past several years.
Winners of the Citizen’s Award
Dr Paul Cromhout and Anche Oosthuizen
Dr Paul Cromhout (on behalf of the Small

Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 40 News Congress news
Anche with colleagues and friends from the Gqeberha Provincial Hospital.
Dr Moti presenting the Humanitarian Award to Dr Cook.
Dr Moti presenting the Citizen’s Award to Dr Cromhout.
Dr Moti presenting the Citizen’s Award to Anche Oosthuize.
Projects Foundation) and Anche Oosthuizen are the 2023 recipients of the Citizen’s Award.
Since 1990, Dr Cromhout has served as CEO of Small Projects Foundation (SPF). Together with SPF, he collaborated with the Transnet Phelophepa Health Train from 1996 to 2006, offering complementary Primary Health Care, including eye care, to one million patients across eight South African provinces. SPF collaborates with various partners to enhance eye care in the Eastern Cape. They’ve trained nurses in eye care and cataract identification, operated on cataract patients in partnership with several organisations, and addressed diabetic retinopathy in Buffalo City with Roche Pharmaceuticals’ support.
In 2022, they initiated the Umbono Project, focusing on eye care for children aged 0-18. Led by optometrists Mita Pema Keshaw, Johan van der Merwe, and Gillian Sangerhaus, the project trained school health teams and community members to improve eye care identification and referral. They screened children from 175 schools, provided prescription spectacles as needed, and partnered with private-sector optometrists and ophthalmologists for testing and prescriptions. Additionally, they support the Bona uBuntu low vision intervention for children, funded by Royal Dutch Visio. Key partners and supporters include Essilor, the African Eye Institute, Lions clubs, Rotary Club of Gately, and the Eastern Cape Departments of Education and Health as key partners and supporters.
Anche Oosthuizen began her optometric career in Grahamstown, Eastern Cape, after graduating from the University of the Free State. In 2019, she relocated to Gqeberha, where she worked at the Port Elizabeth Provincial Hospital’s eye clinic. There, she collaborated with and learned from colleagues in Ophthalmology and Optometry.
She oversaw walk-in patient screenings at the eye clinic, gaining proficiency in basic ophthalmic diagnostics and ocular pathology. As one of only two optometrists in public service in Gqeberha, she recognised the demand for more accessible and affordable eye care services.
She participated in the Bona uBuntu programme’s steering committee, which initiated screening, early intervention, and rehabilitation services for children with low vision in the Eastern Cape. Additionally, she contributed to the Accessibility Panel at the Vision 2023 Low-Vision Conference in Denver, Colorado. Currently, she is pursuing a Master of Health Sciences in Optometry at the University of Johannesburg.

REGISTRARS
Dr Graeme Knight received the Registrar Award for the best oral presentation which was sponsored by Bayer Healthcare to an International Ophthalmology Congress.
Dr Clarise Snyman, won best poster presentation for Outcome of an intra-orbital echinococcal cyst excision using an orbital craniotomy approach.




Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 41 Congress news
Left to right: Dr Farah Moti, previous President of OSSA, Lionel Dobell, Eylea Brand and Customer Lead for South East West Africa and Dr Clarise Snyman, best poster presentation.
Register @ : https://dipity co za/form-view/6 S A G S 2024 CONGRESS Dr Hari Jayaram Dr Winnie Nolan Our Speakers 24-26 MAY CAPITAL ZIMBALI, KZN For more information visit: www sags co za Workshop ECP & AB INTERNO CANAL BASED SURGERIES LIMITED SPACES AVAILABLE
Left to right: Dr Graeme Knight, best oral presentation, Dr Farah Moti, previous President of OSSA and right Lionel Dobell, Eylea Brand and Customer Lead for South East West Africa.

a new 1-piece hydrophobic acrylic IOL with hydroxyethyl methacrylate. J Cataract Refract Surg. 2020 May;46(5):682-687. 6. AcrySof® IQ Vivity® Extended Vision IOL Directions for Use. 7. Bala, Chandra, et al. Multi-country clinical outcomes of a new nondiffractive presbyopia-correcting intraocular lens. Journal of Cataract and Refractive Surgery Publish Ahead of Print DOI:10.1097/j.jcrs.0000000000000712. 8. Varma, Devesh, et al. Clinical Outcomes of a New Non-Diffractive Presbyopia-Correcting Intraocular Lens From Two Large Confirmatory Studies. American Academy of Ophthalmology. Abstract: PA005. 9. Alcon Data on File, 2022. [A01970-REP-211731] - Optical Equivalency of Clareon Monofocal and AcrySof IQ IOL Version 1.2 REF15172. Based on Studies with AcrySof®; Clareon® and AcrySof® are optically equivalent with the same -0.2µm aspheric design resulting in improved depth of focus. Please refer to relevant product direction for use for list of indications, contraindications and warnings. Alcon Laboratories (SA) (Pty) Ltd. Reg No.: 1977/000460/07. Magwa Crescent West, Waterfall City, Jukskei View, Midrand, Johannesburg, 2090. Telephone: 011 840 2300 GSA-CLI-2300001
TIME * CLAREON® MONOFOCAL
**Scan here for references 120000352-03-ALCON-MONOFOCALS-KV--QR--CMYK-PORT.indd 1 01/02/2023 14:04 References: 1. Clareon® IOL Directions for Use. 2. Lane S, Collins S, Das KK, Maass S, Thatthamla I, Schatz H, Van Noy S, Jain R. Evaluation of intraocular lens mechanical stability. J Cataract Refract Surg. 2019 Apr;45(4):501506. 3. Alcon Data on File, 2017. 4. Lehmann R, Maxwell A, Lubeck DM, Fong R, Walters TR, Fakadej A. Effectiveness and Safety of the Clareon Monofocal Intraocular Lens: Outcomes from a 12-Month Single-Arm Clinical Study in a Large Sample. Clin Ophthalmol. 2021;15:1647-1657. 5. Oshika T, Fujita Y, Inamura M, Miyata K. Mid-term and long-term clinical assessments of
SHOCKINGLY BRILLIANT DISTANCE VISION, TIME AFTER
�OLs give your patients functional intermediate vision while maintaining exceptional sharp, crisp distance vision.** But what they see might surprise them.
References: 1. ARGOS User Manual Please refer to relevant product Operator manual for complete list of indications, contraindications and warnings. Alcon Laboratories (SA) (Pty) Ltd. Reg No.: 1977/000460/07. Magwa Crescent West, Waterfall City, Jukskei View, Midrand, Johannesburg, 2090. Telephone: 0118402300. GSA-ARB-2300005
ARGOS® Biometer with Image Guidance by Alcon SMART SOLUTION FOR CATARACT SURGERY PLANNING.
South African & Africa congresses and meetings 2024



MAY
South African Glaucoma Society (SAGS)
Congress 2024
OPTICAL tr.
Your partner in vision since 1989

We are proud to announce the new multipurpose which combines the complete REVO 80 functionality with a Fundus Camera. A single versatile device featuring high resolution OCT and true color fundus imaging for time and space efficiency.

The built-in 12.3 Mpix camera guarantees excellent image reproduction. REVO FC meets all requirements for modern optical tomographs.
The combination of an All in One OCT technology with a Full Color Fundus Camera in one compact system gives you high quality OCT images and a detailed color image for a multipurpose diagnosis.
Tel 012 370 4175 or contact Ahmed 082
414 1472 or Faisal 079 242 2817
Email sales@eurotechoptical.com
cc copy rihaz@eurotechoptical.com
Fax 086 551 3943
Date: 24-26 May 2024
Venue: Zimbali Resort, KwaZulu-Natal
Website: https://www.sags.co.za
JUNE
South African Vitreoretinal Society (SAVRS) Congress
Date: 14-17 June 2024
Venue: Kwa-Maritane Lodge, Pilanesberg
National Park, North West Province
Website: https://www.savrs.co.za
International conference on Ophthalmology and Retinal Disease treatments
Date: 19 Jul 2024
Venue: Durban, South Africa
Website: https://www.iirst.com/event/index.
php?id=2390415
NOVEMBER
International Conference on Retinoblastoma and Retinal Disorders
Date: 04-05 November 2024
Venue: Cape Town, South Africa
Website: https://waset.org/

Visit https://newmedia.b2bcentral.co.za/cd50b100-536e-40f9-adf8147c6bad0b09/index.html to update your calendar
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 44 Events
Photo credit: Shutterstock.com
International congresses and meetings 2024

MAY
Retina World Congress
Date: 9-12 May 2024
Venue: Marriott Harbor Beach, Fort Lauderdale, Florida
Website: https://retinaworldcongress.org/ congress-information/2024-retina-worldcongress/
JUNE
33rd International Conference on Insights in Ophthalmology
Date: June 20-21 2024
Eye to the Future: Ophthalmology Compensation Trends
Venue: Dublin, Ireland
Website: https://ophthalmology. insightconferences.com/speaker-guidelines.php
JULY
International Conference on Retinoblastoma and Retinal Disorders
Date: July 08-09, 2024
Venue: Corfu, Greece
Website: https://waset.org/retinoblastomaand-retinal-disorders-conference-in-july2024-in-corfu
AUGUST
International Conference on Corneal Diseases and Ophthalmology Practice
Date: 30-13 August 2024
Venue: Sydney, Australia
Website: https://waset.org/ conferences-in-august-2024-in-sydney/ program
SEPTEMBER
International Conference on Advances in Ophthalmology Surgery ICAOS
Date: 09-10 September 2024
Venue: Singapore
Website: https://waset.org/advances-inophthalmology-surgery-conference-inseptember-2024-in-singapore
OCTOBER
International Conference on Orbital Trauma and Retinal Detachment
Date: 21-22 October 2024
Venue: London, United Kingdom
Website: https://waset.org/orbital-traumaand-retinal-detachment-conference-inoctober-2024-in-london
NOVEMBER
International Conference on Vision and Ophthalmology
Date: 11-12 November 2024
Venue: Rome, Italy
Website: https://waset.org/vision-andophthalmology-conference-in-november-2024in-rome

August 8

OPTICAL
Your partner in vision since 1989 Designed for complete versatility and ultimate performance, LIGHTLas TruScan Pro V2.0 is the only laser in its class with a choice of four customizable wavelength options.

LIGHTLas TruScan Pro V2.0 is the only modular laser on the market that allows a physician to obtain the system in single-wavelength form and add up to 3 additional wavelengths in the future. Available wavelengths include green 532nm, yellow 561nm or 577nm, red 670nm and infrared 810nm.
LIGHTLas TruScan Pro V2.0 increases treatment speed, safety, and convenience with a large selection of scanning patterns. Enhance conventional treatment outcomes and your patients’ comfort levels with the fastest scanning system on the market.
Available with a choice of up to 4 customizable wavelengths, the LIGHTLas TruScan Pro V2.0 is designed for traditional use or highly specialized needs in all types of clinical settings. Its modular, compact and portable console helps meet and exceed treatment goals.
Tel 012 370 4175 or contact Ahmed 082 414 1472 or Faisal 079 242 2817
Email sales@eurotechoptical.com
cc copy rihaz@eurotechoptical.com
Fax 086 551 3943
Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 45 Events
Photo credit: Shutterstock.com
World Ophthalmology Day
LATANOPROSTENE BUNOD:
An innovative ophthalmic

solution for lowering IOP
The South African launch of latanoprostene bunod ophthalmic solution 0.024%, a novel prostaglandin (PGA) F2 α analogue eye drop, marks a significant milestone in the treatment of patients living with primary open-angle glaucoma (OAG) and ocular hypertension (OHT). Notably, latanoprostene bunod is the first novel molecule approved for these conditions in the past two decades. Join us on this journey as we explore the distinctive features, benefits, and the transformative impact that latanoprostene bunod brings to those living with primary OAG – including normal-tension glaucoma (NTG), the most common subgroup of primary OAG - and OHT.
NTG - also known as low-pressure glaucoma - is defined as a form of primary OAG characterised by mean intraocular pressure (IOP) ≤21mmHg on diurnal testing. The consequences of NTG vary, spanning from non-progressive, asymptomatic conditions to bilateral blindness. A compromised visual field (VF) has a significant effect on an individual’s quality of life. 1
Prevalence of NTG
In individuals of European descent, the prevalence of primary OAG is ~2%, of which ~30% of cases are attributed to NTG.
In sub-Saharan African populations, the prevalence of primary OAG is higher (3% to 8%), with ~50% of cases attributed to NTG. In Asian population, ~95% of primary OAG is attributed to NTG. 1
What causes NTG?
Various theories have been proposed to elucidate the pathogenesis of NTG. While heightened IOP is the main contributor to advancing visual impairment in the majority of primary OAG cases, NTG is a complex condition with multiple potential causes leading to a shared ultimate outcome of retinal ganglion cell loss.1
Despite IOP within the statistically normal
range, evidence suggests the involvement of an IOP-dependent mechanism in many NTG cases, which include vascular insufficiency at the optic nerve head (ONH), metabolic and neurodegenerative disorders, oxidative stress, and abnormal biomechanics of the lamina cribrosa. 1
Genetic factors play a significant role. Family history is a risk factor for the development of NTG. Although rare, three Mendelian genes: Optineurin, tumour necrosis factor receptor-associated binding kinase, and myocilin, have been linked to NTG, with a higher likelihood in cases featuring early onset and a family history. 1
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 46 Normal-tension glaucoma
Photo credit: Shutterstock.com
This is reprinted courtesy of Specialist Forum.

How is NTG diagnosed?
Diagnosing NTG requires excluding all forms of secondary glaucoma, including the potential misclassification of primary OAG. The distinction between NTG and primary OAG relies on eliminating elevated IOP values or spikes, which may not always be discernible with current tonometric methods measuring IOP. 2
Several factors can contribute to an underestimation of IOP, potentially leading to misclassifying patients as NTG rather than primary OAG. These factors include diurnal, nocturnal, and postural IOP variations, with the risk of overlooking peaks, particularly at night.2
Variations in corneal thickness may also play a role, as thin corneas can result in underestimated IOP measurements by various tonometers, including the Goldmann applanation tonometry (GAT). 2
Additionally, corneal biomechanics variations, particularly in more deformable corneas, can lead to underestimated IOP readings by various tonometers, including GAT. 2
While NTG is considered a subtype of primary OAG, distinctive clinical features, beyond IOP values, may assist in differentiation. These features include older age, a higher prevalence of females, the Asian population, and high myopia. 2
Patients living with NTG often exhibit neuro-retinal rim damage in the inferotemporal quadrant, a narrower neuro-retinal rim for a given VF defect, more frequent disc haemorrhages, focal defects in the lamina cribrosa as mentioned, and the beta peri-papillary zone. 2
Patients living with NTG also tend to have more frequent focal areas of cupping at the disc margin, VF defects closer to the fixation point, deeper and more focal defects, and a higher likelihood of association with systemic diseases inducing ischaemic and/or hypoxic damage of the ONH. 2



47 Normal-tension glaucoma
2024 Congress JEREMIAH TAO INTERNATIONAL SPEAKER 27-29 SEPT Le Franschhoek Hotel & Spa, Franschhoek www.sasops.co.za www.sasops.co.za
Implicated systemic diseases include migraine, Raynaud’s phenomenon, primary vascular dysfunction (Flammer syndrome), systemic hypotension (especially nocturnal arterial hypotension), and obstructive sleep apnoea syndrome. A comprehensive systemic evaluation for potential contributing diseases as mentioned above, is crucial, particularly in cases of disease progression resistant to IOPlowering therapy. 2
Efficacy of lowering IOP to delay progression and prevent VF damage
Numerous clinical trials have proven the efficacy of lowering IOP in delaying the progression, and potentially preventing the onset of VF damage, in patients living with NTG. The findings of these studies stresses the pivotal role of IOP reduction in NTG therapy and provides a compelling rationale for further reducing pressure in cases displaying disease progression despite achieving low IOP values. 2
The Collaborative Normal-Tension Glaucoma Study, a comprehensive multicentre trial involving patients living with NTG and documented VF damage progression, revealed that, over a seven-year period, an IOP reduction of ≥30% led to stabilisation of VF defects in 88% of cases, in contrast to the 65% observed in untreated patients.3
In a randomised double-masked clinical trial known as the Early Manifest Glaucoma Trial, which included patients living with
primary OAG and early VF defects (including 53% with NTG), a 25% reduction in IOP demonstrated a significant impact. After a six-year follow-up, the study group exhibited a reduced risk of disease progression (45%), compared to a higher risk (62%) observed in the control group. 4
These studies collectively suggest that for a significant impact on decreasing the risk of NTG progression, the reduction in IOP should ideally reach at ≥30% or more. 2
How is NTG treated?
Individualised management of IOP reduction is essential, tailored to each patient’s unique needs. Adjustments to the treatment plan may be necessary as the disease progresses or a patient’s response to therapies changes over time.2
Effective management of NTG requires consistent monitoring of IOP, VF, and OHN health. Additionally, incorporating diurnal IOP curves and addressing IOP peaks is a pivotal therapeutic strategies in patients living with NTG - especially when conventional office IOP values remain within the statistically normal range. 2
Given that NTG patients inherently exhibit baseline IOP within the statistically normal range, achieving significant IOP reduction through pharmacotherapy alone may be challenging. Consequently, nonpharmacological interventions, such as laser and surgical treatments, are often considered in the management of NTG. 2
However, the mainstay of NTG treatment
is hypotensive eye drops. Reducing IOP can be achieved by either diminishing aqueous production or enhancing trabecular or uveoscleral outflow pathways. 2
PGA F2 α analogues is the preferred initial treatment for primary OAG and NTG. These drugs exhibit superior efficacy in IOP reduction, ensuring both diurnal and nocturnal IOP control. With a favourable safety profile and the convenience of oncedaily application, PGA analogues also promote adherence to treatment. 2
PGA analogues’ mechanism of action involves reducing IOP by primarily increasing uveo-scleral outflow through ciliary muscle widening. 2
PGA analogues have demonstrated their ability to maintain IOP reduction over a 24-hour period and diminish nocturnal IOP peaks, which are recognised as risk factors contributing to the progression of NTG. 2
Apart from PGA analogues, other treatment options include nitric-oxide (NO)-donating PGAs analogues, beta-adrenergic antagonists, selective alpha-2-adrenergic-agonist, carbonic anhydrase inhibitors, miotics, and Rho-associated protein kinase inhibitors. 2
Latanoprostene bunod : A novel PGA analogue
The first PGA analogue (latanoprost) for the treatment of glaucoma was approved by the American Food and Drug Administration (FDA) in 1996. Latanoprostene bunod ophthalmic solution 0.024% is one of the
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 48 Normal-tension glaucoma
Optic nerve in advanced glaucoma disease.
Photo credit: Shutterstock.com
latest PGA analogues to be approved by the FDA (2017) – the first in almost 20 years.
Latanoprostene bunod was approved in South Africa in November 2022 for the reduction of IOP in patients living with OAG or OHT and was officially launched in January 2024. 2,5,6
Latanoprostene bunod is a novel, innovative IOP-lowering eye drop with a dual mode of action. Upon ocular instillation, latanoprostene bunod undergoes rapid metabolism, yielding latanoprost acid, a F2 α analogue, and butanediol mononitrate, a NO-donating component.7
The latter is subsequently converted into 1,4 butanediol, an inactive metabolite, and NO. Latanoprost primarily reduces IOP by fostering uveoscleral outflow through the long-term remodelling of extracellular matrices within the ciliary body, specifically influencing non-conventional outflow.7
On the other hand, NO donors induce relaxation of the trabecular meshwork and Schlemm’s canal, resulting in augmented aqueous outflow, commonly referred to as conventional outflow.7
Both preclinical and clinical studies indicate that both active components of latanoprostene bunod (latanoprost acid and NO) synergistically contribute to its robust IOP-lowering efficacy.7
Although glaucoma is inherently irreversible, the introduction of latanoprostene bunod signifies a revolutionary strategy to mitigate IOP, one of the hallmarks of a glaucoma. Explore the innovative mechanism driving latanoprostene bunod’s action and discover the transformative potential of this novel addition to the armamentarium for managing glaucoma in this brief video https://vimeo.com/922348831/962ddfc89b.
Latanoprostene bunod consistently shows efficacy in reducing IOP compared to other therapies
Latanoprost has consistently demonstrated efficacy in reducing IOP, making it a valuable option for patients living with primary OAG or OHT. Below we highlight some of the findings from noteworthy studies:8,9,10,11,12 Eveleth et al (2012) conducted a study comparing different doses of latanoprost to evaluate their IOP-lowering effects in patients living with primary OAG or OHT. The study showed no significant differences in IOP reductions irrespective of doses. These results, supported by secondary analyses, demonstrated consistent efficacy across various latanoprost doses. 8
In a comprehensive 52-week study by Kawase
et al (2016), the long-term safety and efficacy of latanoprostene bunod 0.024% were evaluated in patients with various forms of glaucoma. It is noteworthy that about 75% of the study eyes and 86% of the treated fellow eyes had a baseline IOP within the range of 15 to 21 mmHg, suggesting a predominance of subjects with NTG enrolled at baseline. Overall, baseline IOP averaged 19.6 mmHg for the study eye and 18.7 mmHg for the treated fellow eye. Despite the subjects’ low baseline IOP, the study reported significant IOP reductions of 22% and 19.5% in study and treated fellow eyes, respectively at week 4. Reductions greater than 22% and 20% in the study and treated fellow eyes, respectively, were seen at every visit after week 4. At week 52, the reductions in IOP were 26.3% and 23% in the study eyes and treated fellow eyes, respectively, to 14.4 mmHg in both eyes. Latanoprostene bunod exhibited a favourable safety profile, with conjunctival hyperaemia, eyelash growth, eye irritation, and eye pain being the most common mild-to-moderate adverse events.7
Weinreb et al (2015) conducted a doseranging study comparing latanoprostene bunod to latanoprost 0.005%, aiming to identify optimal concentrations for IOP reduction. Latanoprostene bunod 0.024% demonstrated significantly greater reductions in diurnal IOP

A multivitamin and mineral supplement containing a neuroprotective agent, Citicoline ( ) and other nutrients that contribute to:1



















Autumn 2024 • Vol 19 | No 2 SA Ophthalmology Journal 49 Normal-tension glaucoma O er Neuroprotection1 Reference: 1. CEBROLUX™ NF powder for oral solution (package insert). South Africa: Soflens (Pty) Ltd; 2019. Scheduling status: S0 Proprietary name and dosage form: CEBROLUX™ NF powder for oral solution. Composition: Each 3 g sachet contains: Citicoline (Cognizin®) 250 mg, Vitamin C 60 mg, Vitamin B3 12 mg, Vitamin E 8,2 mg, Zinc 6,25 mg, Vitamin B5 4,5 mg, Vitamin B6 1,05 mg, Vitamin B2 1,05 mg, Vitamin B1 0,83 mg, Vitamin A 600 μg, Folic acid 150 μg, Biotin 37,5 μg and Vitamin B12 1,875 μg. Bausch & Lomb Incorporated. CEBROLUXTM is a trademark of Bausch & Lomb Incorporated or its a liates. COGNIZIN® is a registered trademark of KYOWA HAKKO BIO CO., LTD. This unregistered medicine has not been evaluated by the SAHPRA for its quality, safety or intended use. For full prescribing information, refer to the Professional Information. Applicant: Soflens (Pty) Ltd. Reg. No.: 1968/011787/07. 254 Hall Street, Centurion, 0157. Tel: +27 10 025 2100. www. bausch.co.za BL601/22 Oral doses of citicoline are rapidly absorbed with greater than 90% bioavailability1 Normal synthesis & metabolism of some neurotransmitters Normal cognitive function & mental performance Maintenance of normal vision Normal functioning of the nervous system Protection of cells from oxidative stress A NEUROPROTECTIVE AGENT With
compared to latanoprost, without increased ocular hyperaemia. The study concluded that latanoprostene bunod 0.024% was more effective for IOP lowering, marking a significant advancement in glaucoma treatment.9
The efficacy of latanoprostene bunod 0.024% was further demonstrated in a phase 3 study by Weinreb et al (2016), where it was compared with timolol maleate 0.5% over 12 weeks. Latanoprostene bunod 0.024% consistently showed significantly lower mean IOP at all time points compared to timolol. Furthermore, latanoprostene bunod 0.024% achieved higher percentages of patients with mean IOP ≤18mmHg and IOP reduction ≥25% consistently, highlighting its sustained efficacy throughout the study duration. 10
Liu et al (2016) investigated latanoprostene bunod’s diurnal and nocturnal effects compared to timolol 0.5% in patients with early primary OAG and OHT. While both treatments reduced diurnal IOP, latanoprostene bunod 0.024% exhibited a more pronounced nocturnal IOP reduction, indicating its effectiveness during crucial periods. The study also reported increased ocular perfusion pressure with latanoprostene bunod 0.024%, emphasising its potential benefits beyond IOP reduction.11
A recent systematic literature review by Harasymowycz et al (2022) evaluated latanoprostene bunod’s efficacy compared to other glaucoma medications. Latanoprostene bunod consistently outperformed unoprostone and several beta-blockers, showcasing its significant efficacy in reducing IOP. When compared to other PGA analogues, latanoprostene bunod demonstrated superiority over latanoprost, travoprost and tafluprost and comparable performance to bimatoprost 0.01%.12
Take-away messages
1 Consistent efficacy across various latanoprost doses: Latanoprost demonstrates reliable efficacy in reducing IOP

2024 Conference
across different concentrations, providing flexibility in dosage for glaucoma management.
2 Long-term safety and efficacy of latanoprostene bunod: A comprehensive evaluation spanning 52-weeks affirms the enduring safety and efficacy of latanoprostene bunod in patients with diverse forms of glaucoma, including sustained IOP reductions and a favourable safety profile.
3 Significantly greater diurnal IOP reduction without ocular hyperaemia: Latanoprostene bunod stands out by achieving significantly greater reductions in diurnal IOP compared to latanoprost, offering enhanced efficacy without the drawback of increased ocular hyperaemia.
4 Consistently lower mean IOP: Latanoprostene bunod consistently outperforms timolol by showcasing significantly lower mean IOP at all evaluated time points, reinforcing its efficacy throughout the day in patients with primary OAG or OTH.
5 Higher percentages of patients achieving target IOP levels: Latanoprostene bunod surpasses expectations by achieving higher percentages of patients with mean IOP ≤18mmHg and substantial IOP reduction, emphasising its efficacy in effectively managing IOP levels.
6 Pronounced nocturnal IOP reduction and increased ocular perfusion pressure: Latanoprostene bunod’s effectiveness extends to crucial nocturnal periods, displaying a more pronounced reduction in IOP. Additionally, the study highlights an increase in ocular perfusion pressure, suggesting potential benefits beyond traditional IOP reduction.
7 Superior efficacy compared to other glaucoma therapies: In comparative assessments, latanoprostene bunod consistently outperforms unoprostone and several beta-blockers, underscoring its significant efficacy in reducing IOP and positioning it as a promising choice in glaucoma management.
The launch of latanoprostene bunod ophthalmic solution 0.024% represents a groundbreaking advancement in the treatment landscape for South African patients with primary OAG and OHT.
References
1. Gosling D, Meyer JJ. Normal Tension Glaucoma. [Updated 2022 Dec 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm. nih.gov/books/NBK576377/


2. Salvetat ML, Pellegrini F, Spadea L, et al. Pharmaceutical Approaches to Normal Tension Glaucoma. Pharmaceuticals (Basel), 2023.
3. Anderson DR, et al. Normal Tension Glaucoma Study. Collaborative normal tension glaucoma study. Curr Opin Ophthalmol, 2003.
4. Heijl A, Leske MC, Bengtsson B, et al. Early Manifest Glaucoma Trial Group. Reduction of intraocular pressure and glaucoma progression: results from the Early Manifest Glaucoma Trial. Arch Ophthalmol, 2002.
5. Vyzulta. FDA Approval History. 2017. [Internet]. Accessed 7 March 2024. Available at: https:// www.drugs.com/history/vyzulta.html
6. Professional information. Vyzulta. 2022. [Internet]. Accessed 7 March 2024. Available at: https:// pi-pil-repository.sahpra.org.za/wp-content/uploads/2023/01/Final_PI_Vyzulta_Applicant.pdf
7. Kawase K, Vittitow JL, Weinreb RN, Araie M, JUPITER Study Group. Long-term Safety and Efficacy of Latanoprostene Bunod 0.024% in Japanese Subjects with Open-Angle Glaucoma or Ocular Hypertension: The JUPITER Study. Adv Ther, 2016.
8. Eveleth D, Starita C, Tressler C. A 4-week, dose-ranging study comparing the efficacy, safety and tolerability of latanoprost 75, 100 and 125 μg/mL to latanoprost 50 μg/mL (xalatan) in the treatment of primary open-angle glaucoma and ocular hypertension. BMC Ophthalmol, 2012.
9. Weinreb RN, Ong T, Scassellati Sforzolini B, et al. A randomised, controlled comparison of latanoprostene bunod and latanoprost 0.005% in the treatment of ocular hypertension and open angle glaucoma: the VOYAGER study. British Journal of Ophthalmology, 2015
10. Weinreb RN, Scassellati Sforzolini B, Vittitow J, Liebmann J. Latanoprostene Bunod 0.024% versus Timolol Maleate 0.5% in Subjects with Open-Angle Glaucoma or Ocular Hypertension: The APOLLO Study. Ophthalmology, 2016.
11. Liu JHK, Slight JR, Vittitow JL, et al. Efficacy of Latanoprostene Bunod 0.024% Compared With Timolol 0.5% in Lowering Intraocular Pressure Over 24 Hours. Am J Ophthalmol, 2016.
12. Medeiros FA, Martin KR, Peace J, et al. Comparison of Latanoprostene Bunod 0.024% and Timolol Maleate 0.5% in Open-Angle Glaucoma or Ocular Hypertension: The LUNAR Study. Am J Ophthalmol, 2016.
13. Harasymowycz P, Royer C, Cui AX , et al. Short-term efficacy of latanoprostene bunod for the treatment of open-angle glaucoma and ocular hypertension: a systematic literature review and a network meta-analysis. British Journal of Ophthalmology, 2022.
Vol 19 | No 2 • Autumn 2024 SA Ophthalmology Journal 50 Normal-tension glaucoma R E G I S T E R @ W W W S A V R S C O Z A 14 - 17 JUNE I n t e r n a t i o n a l S p e a k e r DR JAY SRIDHAR K W A M A R I T A N E B U S H L O D G E , P I L A N S B E R G








GLAUCOMA works on
nerves causing tunnel vision, it’s time to reduce IOP 1,2 TEST • TREAT • PRESERVE sight References: 1. Weinreb RN, Aung T, Medeiros FA. The pathophysiology and treatment of glaucoma: a review. JAMA. 2014; 311(18):1901-1911. 2. South African Glaucoma Society. Glaucoma algorithm and guidelines for glaucoma, 2016. Available at https://www.sags.co.za/doctors-information. Accessed 12 December 2019. S4 TRAVATAN® Eye Drops, solution (0,004 %), 40 μg of travoprost per ml in a sterile ophthalmic solution, 36/15.4/0333, Novartis SA (Pty) Ltd. S3 SIMBRINZA® 10 mg/ml + 2 mg/ml eye drops, suspension. Reg. No.: 50/15.4/0358. Each ml contains 10 mg of brinzolamide and 2 mg of brimonidine tartrate. S4 DUOTRAV® eye drops, 1 ml of solution contains 40 μg travoprost and 6,83 mg timolol maleate equivalent to 5 mg timolol, A40/15.4/0511, Novartis SA (Pty) Ltd. S3 AZOPTIC® Eye Drops (Suspension), 10 mg brinzolamide per ml, 34/15.4/0382, Novartis SA (Pty) Ltd. S4 AZARGA® eye drops, suspension, 1 ml of suspension contains 10 mg brinzolamide and 5 mg timolol (as timolol maleate), 44 /15.4/0046, Novartis SA (Pty) Ltd. Note: Before prescribing, consult full prescribing information. 1 DUOTRAV® TRAVATAN® AZARGA® AZOPTIC® Scan the QR codes to view the full Professional Information for the products below: IOP = Intraocular pressure SIMBRINZA® Novartis South Africa (Pty) Ltd. Magwa Crescent West, Waterfall City, Jukskei View 2090. Tel. +27 11 347 6600. Co. Reg. No. 1946/020671/07. Novartis Adverse Drug Reaction Reporting: Email: patientsafety.sacg@novartis.com. Web: https://psi.novartis.com/. Tel: 0861 929-929. Fax: 011 929-2262. Marketed and Distributed by Adcock Ingram Holdings Limited. 1 New Road, cnr 7th Road, Midrand, 1685. Tel: 0860 ADCOCK (232625) Co. Reg. No. 2007/019928/07. ZA2304173788 Exp.: 04/2025 43635 04/23
When
the








WE’VE GOT FOUR SOLUTIONS
REFERENCE: 1. European Glaucoma Society Terminology and Guidelines for Glaucoma, 5th Edition. Br J Ophthalmol. 2021. GANFORT® Eye Drops. Contains bimatoprost 0,3 mg/ml and timolol maleate equivalent to 5 mg/ml timolol. Registration No.: 42/15.4/0127. ALPHAGAN® Purite® Eye Drops. Contains brimonidine tartrate 1,5 mg/ml. Registration No.: A39/15.4/0202. COMBIGAN® Eye Drops. Contains brimonidine tartrate 2,0 mg/ml and timolol maleate equivalent to timolol 5,0 mg/ml. Registration No.: A39/15.4/0464. LUMIGAN® 0,01% Eye Drops. Contains bimatoprost 0,1 mg/ml. Registration No.: 42/15.4/0835. AbbVie (Pty) Ltd, Reg. 2012/068113/07. Address: Building 7, Waterfall Corporate Campus, 74 Waterfall Drive, Midrand, 1685, South Africa. Tel: 011 031 1600. For full prescribing information refer to the professional information approved by the medicines regulatory authority, accessible by e-mailing medicalinfo.za@abbvie.com. For adverse events, report to MEAPV@abbvie.com. Date of Publication of this material: March 2024. Promo. No. ZA-GAN-240004. STARTOPTION ADD-ONOPTION STEP-UPOPTION ADD -ONOPTION SHORT-TERM TREATMENT LUMIGAN® 0,01% Bimatoprost 0,1 mg FIXED COMBINATION GANFORT® Bimatoprost 0,3 mg/Timolol 5,0 mg COMBIGAN® FIXED COMBINATION Brimonidine Tartrate 2,0 mg/Timolol 5,0 mg ALPHAGAN® PURITE® NON-PROSTAGLANDIN Brimonidine Tartrate 1,5 mg 1 4 2 3
PRESERVING SIGHT STARTS WITH A DROP1
The complexity of glaucoma requires treatments tailored to meet patients’ needs at every stage of their disease1



























































































































































































